
Biopolymer gels as a basis of cryoprotective medium
for testicular tissue of rats
Nataliia Volkova
.
Mariia Yukhta
.
Anatoliy Goltsev
Received: 10 September 2018 / Accepted: 17 November 2018
Ó Springer Nature B.V. 2018
Abstract Cryopreservation of testis tissue is a
promising approach to save fertility in prepubertal
boys under going gonadotoxic cancer therapies. The
using biopolymers as a basis of cryoprotective
medium can be effective for the optimization of
cryopreservation protocols of immature testicular
tissue. The research purpose was to determine mor-
phological parameters and metabolic activity of
seminiferous tubules of immature rat testes under
exposure to cryoprotective solution (DMSO) based on
collagen or fibrin gels (CG or FG) as one of the first
stages of developing the cryopreservation protocol. It
was found that 30-min exposure of tissue samples to
CG and FG with 0.6 M DMSO did not impair the
spermatogenic epithelium and metabolic activity of
the cells (MTT test and total lactate dehydrogenase
activity). The use of FG at the time of exposure of
45 min did not lead to significant changes in the
metabolic activity in contrast to other groups. The
findings could be used to substantiate and develop the
effective techniques for cryopreservation of immature
seminiferous tubules.
Keywords Immature testicular tissue
Spermatogenic epitelium Cryoprotective medium
Collagen gel Fibrin gel Metabolic activity
Introduction
In prepubertal boys affected by cancer, cryopreserva-
tion of testicular fragments prior to gonadotoxic
treatment is an ethically accepted strategy to save
fertility (Abrishami et al. 2010; Michele et al. 2017).
Frozen immature testicular tissue can later be used for
completion of spermatogenesis after in vitro matura-
tion, germ cell transplantation or testicular tissue
grafting (Travers et al. 2011). Despite the fact that the
low temperature preservation of testis is associa ted
with some difficulties (different cell size, post thawing
ischemia), it can allow to preserve the different
testicular cells in their ‘‘niche’’ with respect interac-
tions between germ cells and Sertoli cells (Milazzo
et al. 2010). Nevertheless, the optimization of cryop-
reservation protocols for improving recovery has not
been practically investigated. In our opinion, the
various biotechnological methods using natural
biopolymers can be effective for this purpose, but
they require additional studies.
Tissue fragment encapsulation is based on cell
scaffold technology (Nicodemus and Bryant 2008).
The latter relies on immobilization of cells in a
N. Volkova (&) M. Yukhta A. Goltsev
Department of Cryopathophysiology and Immunology,
Institute for Problems of Cryobiology and Cryomedicine
of the National Academy of Sciences of Ukraine, Kharkiv,
Ukraine
e-mail: [email protected]
123
Cell Tissue Bank
https://doi.org/10.1007/s10561-018-9740-z
(0123456789().,-volV)(0123456789().,-volV)

biomaterial which allows bidirectional diffusion of
nutrients, oxygen, and waste, thus promoting cell
interactions. Substances for encapsulation should also
be biocompatible and favor (subject to application)
vascular invasion and tissue integration in the host
(Dhandayuthapani et al. 2011; Jafari et al. 2017).
Polymers are very often used as scaffolds. They can
be synthetic or biological origin, and degradable or
non-degradable. The two main classes of natural
biopolymer are proteins and polysaccharides (Guarino
and Ambrosio 2014; Jafari et al. 2017).
Collagens are most abundant family of the complex
enzymatically degradable proteins that has unique
biological properties . They are involved in forming of
membranes, fibrillar system and other components of
the extracellu lar matrix, dete rmining structural integ-
rity and physiological functions in cells (Gelse et al.
2003). They have been oftentimes applicated for
biomedical investigation (Nair and Laurencin 2007;
Bret et al. 2011). The studies in field of cryobiology,
conducted by Allenspach and Kraemer (1989) showed
that the distribution of water and structure of ice
crystals in collagen gels depend on the composition of
the gel and the program of freezing. Thus, the presence
of an extracellular matrix may affect the structure of
ice formation during freezing-warming, and hence the
final result of cryopreservation.
Now, using of fibrin gel is widespread as an
alternative to collagens. The products derived from
autologous blood are used in the regenerative
medicine for the recovery of damaged tissues and
organs. One of the important properties of fibrin gel is
ability to degrade in a controllable method and self-
organizations into a polymer system that imitates of
blood coagulation. At the same time, fibrin gel and
other derivatives of blood (serum, plasma) contain a
large number of different bioactive substances that
used as protectors from the damages during the
cryopreservation of the cells (Nair and Laurencin
2007; Li et al. 2015).
The apply of gels as a matrix for fragments of the
tissue of testicles will allow to minimize tissue damage
during cryopreservation by reducing the amount of
free water and increasing of tissue resistance to
overcooling. In addition, it is known that biopolymer
gels have high viscosity which makes them capable to
influence the proce sses of ice crystal formation
inhibiting their growth in volume and thus reduce
the degree of mechanical action on the tissues (Koebe
et al. 1990; Takahashi et al. 1988).
In our preliminary study, it has been shown that a
30-min incubation of samples of tubules of immature
rat testes in Hanks medium with add of 5% BSA and
0.6 M DMSO did not cause change to the morpho-
logical characteri stics of tissue and metabo lic param-
eters of cells (Volkova et al. 2017). The aim of this
research was to determine morphological and func-
tional parameters of seminife rous tubules of immature
rat testes under exposure to cryoprotective solu tion
(DMSO) based on collagen and fibrin gels as one of
the stages in optimizing of the cryopreservation
protocol.
Materials and methods
Animals
Outbreed white sexually immature male rats
(50 ± 15 g weight, aged 7–8 weeks, n = 50) were
used in the study. All the manipulations were carried
out in accordance to the European convention for the
protection of vertebrate animals used for experimental
and other scientific purposes (Strasbourg,
18.III.1986). The protocols were approved by the
Bioethics Committee of Institute for Problems of
Cryobiology and Cryomedicine of the NAS of Ukraine
(Permit No 2016-05).
Preperation of biopolymer gels
Collagen gel (CG) was prepared using collagen type I,
which was obtained from rat tendons as described by
Chandrakasan et al. (1976). The pH was brought to
neutral using 0.34 N NaOH solution.
Fibrin gel (FG) was received from the fresh
autologous blood of animals, which was obtained
from a cardiac vein and centrifuged for 12 min at a
rate of 1000 g. After centrifugation, three fractions of
blood were received: the lower fraction was erythro-
cyte mass; the upper fraction was platelet-poor
plasma, and the medium one was platel et-rich fibrin
gel. Only blood samples without signs of hemolysis
were used in the work.
123
Cell Tissue Bank
Cryoprotective media
We used DMSO (PanEco, Russia) as the widespread
cryoprotectant for testicular tissue freezing at final
concentrations of 0.6 M (Keros et al. 2007), but
cryoprotective media differed in the basis. The first
experimental medium (CG ? DMSO) consisted of
collagen gel with 0.6 M DMSO, and the second one
(FG ? DMSO) consisted of fibrin gel with
0.6 M DMSO. The controls were corresponding gels
without cryoprotectant (CG and FG respectively) and
intact samples of testicular tissue.
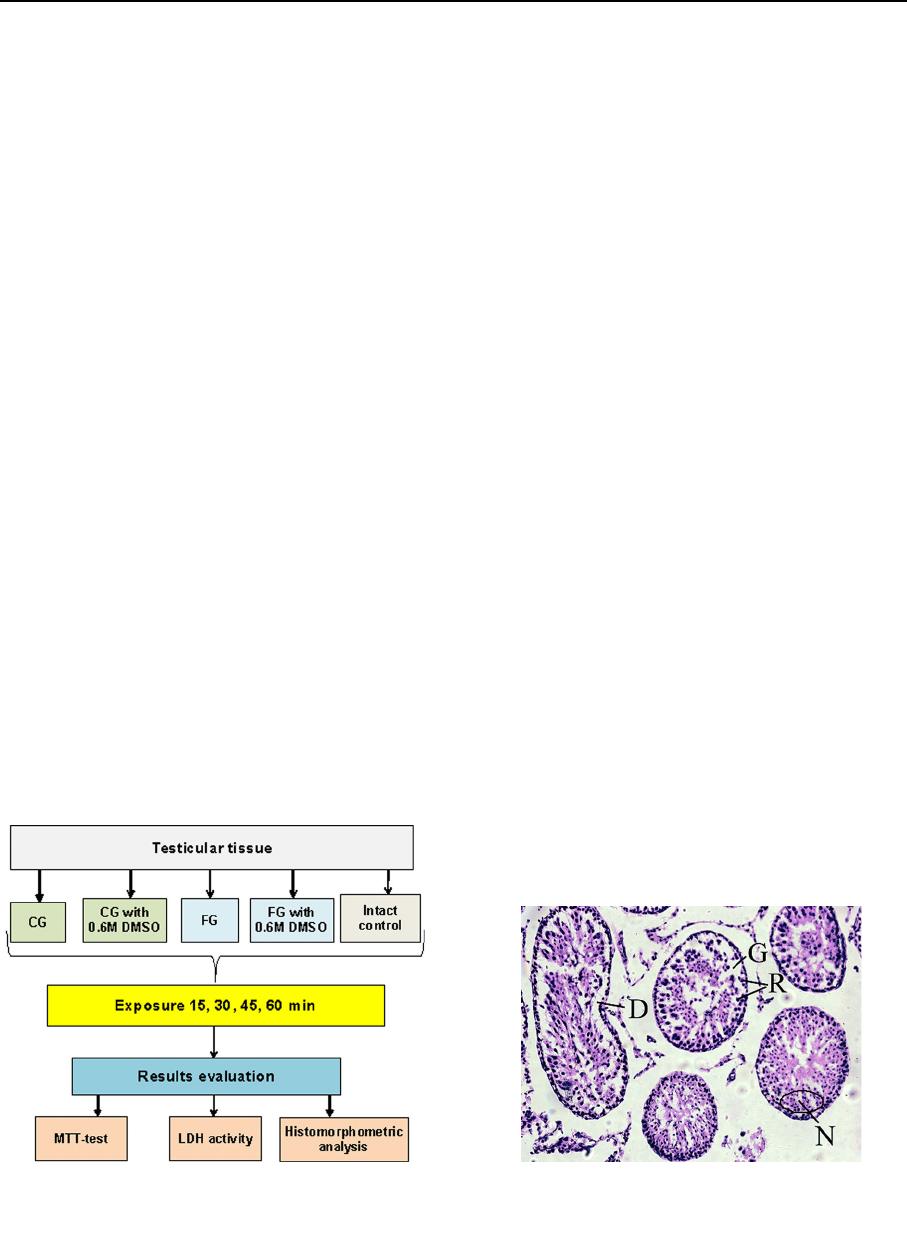
Exposure of testicular tissue
Samples of testicular tissue weighing 75 ± 5 mg were
isolated mechanically immediately before the study.
They were immersed in the cryoprotective media and
exposed during 15, 30, 45 and 60 min at 4 °C (Keros
et al. 2007; Milazzo et al. 2010; Travers et al. 2011).
The samples after exposure were (washe d out by a
three-step change of Hanks solution and used for
morphological and biochemical studies (Fig. 1).
MTT test
Quantification of metabolic activity was executed at
15, 30, 45 and 60 min of term expositions on testicular
tissue samples. MTT test is based on the ability of
dehydrogenases to restore [3-(-4,5-dimethylthiazolyl-
2)-2,5-diphenyltetrazolium bromide] in living cells to
blue crystals of formazan, which are insoluble in
water. A final concentration of 0.5 mg/mL MTT
(Fluka, Germany) was added to the samples and
incubated for 3 h at 37 °C. Then the medium was
deleted and 100% DMSO was added to each samples
of tissue to solubilize the precipitate (Mossman 1983).
Absorbance was read at 570 nm.
Analysis of total lactate dehydrogenase (LDH)
activity
The total LDH activity was estimated quantitatively
by the method of UV spectrophotometry was using
test kits (Felicit, Ukraine). The samples of tissues of all
experimental groups were homogenized and filtered,
with following centrifugation (1000 g for 10 min).
Reaction was started by the addition of enzyme sample
to 20 lL supernat ant of tissue homogenat with
following spectrophotometrical measuring of a
decrease in absorbance of NADH at 365 nm.
Histomorphometry
Histomorphometry was carried out maintaining blind-
ing by involving the third person who did not take part
in the experiment. Paraffin blocks were prepared and
slices of 7 lm thickness were stained by hematoxylin
and eosin. Histological preparations were studied
using Axio Observer Z1 inverted microscope (Carl
Zeiss, Germany). Obtained image files were processed
using the Axiovision v. 4.8 (Carl Zeiss).
The following qualitative criteria were studied: cell
retraction, nuclei condensation, cell detachment and
formation of gaps (Fig. 2). Additionally, a cell density
Fig. 1 Experimental scheme. CG collagen gel, FG fibrin gel,
DMSO dimethyl sulfoxide, MTT 3-(4,5-dimethylthi azol-2-yl)-
2,5-diphenyltetrazolium bromide, LDG lactate dehydrogenase
Fig. 2 Histological evaluation of spermatogenic epithelium
damages. R cell retraction, N nuclei condensation, D cell
detachment, G formation of gaps
123
Cell Tissue Bank
of spermatogenic epithelium was detected as the
average of the numb er of nuclei in the section area
(0.102 mm
2
) with following conversion per 1 mm
2
.
Statistical analysis
The Mann–Whitney U-criterion was used to determine
the statistical significance of the differences in
continuous variables when comparing between the
groups with multiple (more than two) comparisons
Kruskal–Wallis ANOVA tests using Excel (Microsoft,
USA) and Statistics 8 (StatSoft, USA) software.
Results
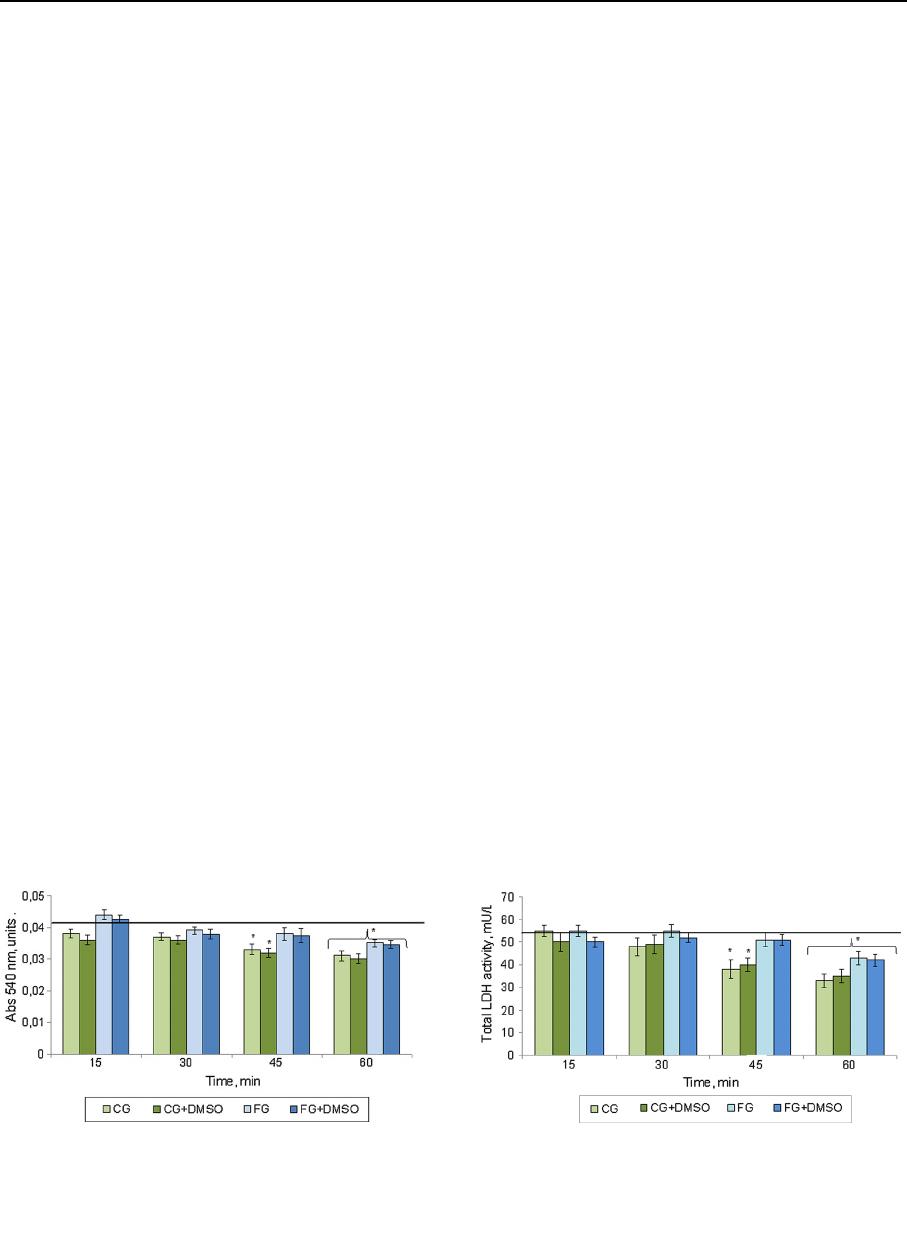
Metabolic activity
The obtained experimental dates of the MTT test
indicate that the 15- or 30-min exposures in all studied
media did not lead to a significant change in metabolic
activity of the immature testis tissue if compared to the
intact control and between the investigated biopoly-
mers (Fig. 3). In addition, the metabolic activity was
decreased in groups with CG by 19.5% and CG ?
DMSO by 21.9% compared to intact control at the
45 min. In the media based on FG this parameter
remained at the level of intact control. After 60 min of
exposure the investigated index in all cases was lower
if compared to intact control: for CG and CG ?
DMSO by 37.5 and 32.7%, for FG and FG ? DMSO
by 17.3 and 19.2% respectively.
The results of the study of the influence of
cryoprotective media on the total LDH activity are
shown in Fig. 4. The time of exposu re of 15 and 30
min in all tested biopolymers media did not lead to
strong changes in LDH activity if compared to the
intact control as well as between the investigated
media. At the 45-min exposure there was a tendency to
decrease the investigated index in the CG and
CG ? DMSO media by 17.2 and 18.3% respectively
compared to the intact control. At 60-min exposure of
tissue in media based on CG, we observed a decrease
in LDH act ivity index by more than 1.5 times if
compared to intact control. Thus, the increase in the
tissue exposure time in the media on base of CG more
than 30 min resulted in significant changes in LDH
activity, which probably indicated the violation of
metabolic processes in the cells. The use of FG at the
timing of exposure of 15–45 min did not lead to
significant changes in this parameter. The data of the
LDH activity on the 60th minute of observation are
consistent with the results of MTT test at the same
time, which indicates the inhibition of metabolic
processes in experimental samples due to the exposure
duration.
Histomorphometry
Dates of the biochemical study showed no influence of
investigated biopolymers on seminiferous tubules of
testes under the 30-min term of exposure. Significant
changes were noted starting from 45 min. Therefore
the next step was histomorphometric analysis to obtain
Fig. 3 Dynamic of metabolic activity (MTT test) of seminif-
erous tubules of immature rat testes under exposure to
investigated media. Solid line is an index of intact control.
*The difference is statistically significant relative to intact
control (M ± m; n = 5; p \ 0.05)
Fig. 4 Dynamics of total LDH activity of seminiferous tubules
of immature rat testes under exposure to investigated media.
Solid line is an index of intact control. *The difference is
statistically significant relative to intact control (M ± m; n = 5;
p \ 0.05)
123
Cell Tissue Bank
more information about structural characteristics at
this term of exposure (Fig. 5).
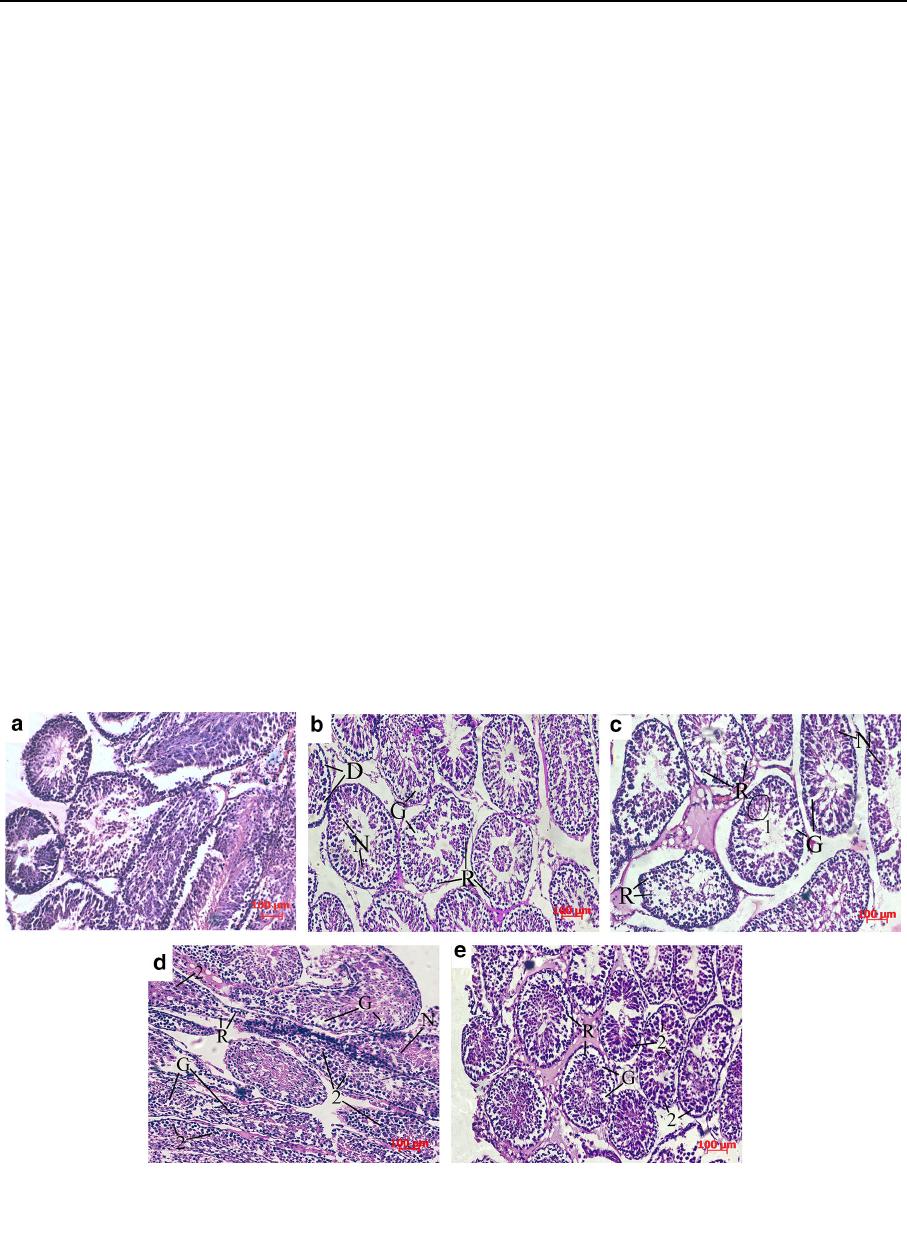
It was found that seminiferous tubules of the intact
group had a form of the rounded formations of the
basal membrane inside of which there was a multilayer
germinative epithelium (Fig. 5 Intact control). The
population of spermatogenic epithelium was repre-
sented by all types of spermatogenic cells excepted of
spermatozoa. Spermatogonia were located on the
basal membrane, above them, spermatocytes were
placed in 2–3 rows. In some tubules, the cells reached
a developmental level of early spermatids.
After 30-min exposure in CG (Fig. 5 CG), in the
most case s, there was slight desquamation of epithe-
lium into the lumen of seminiferous tubules. The
detachment of sperma togenic epithelium from the
basal membrane was partial. In addition, there was a
chaotic location of the germinative cells with forma-
tion of gaps and ruptures inside the spermatogenic
epithelium due to the obvious cell retraction. Nuclei
condensation was slightly present.
In the case of CG ? DMSO combination, germi-
native cells in most cases saved their connection with
the basal membrane and orientation within the sper-
matogenic epithelium despite the obvious retraction
and gap formation. However, there were extensive
regions covered by karyolysis (the cell nuclei are
swollen, slightly colored with hematoxylin, without
distinct contours) in the center of separate seminifer-
ous tubules. At the same time, the tubules were
detected in which spermatocytes had a hyperchromic
nucleus and a sharp eosinophilic cytoplasm (Fig. 5
CG ? DMSO).
When FG was used, cell nucl ei of the primarily
spermatocytes had a swollen appearance and were
sharply hyperchromic. The contours of seminiferous
tubules were fuzzy and deformed but desquamation
phenomena were absent. The cytoplasm of germina-
tive cells was slightly retracted (Fig. 5 FG).
After exposure in FG ? DMSO, as well as in the
previous case, cell nuclei of the spermatogenic
epithelium in some seminiferous tubules had a swollen
appearance and were sharply hyperchromic. But it
should be noted that the FG, in contrast to the CG, had
a more pronounced protective effect in combination
with DMSO, preventing the development of necrosis
in germinative cells (nuclei condensation was slightly
present). Cell retraction and formation of gaps in most
cases were slightly present too and cell detachment
was absent (Fig. 5 FG ? DMSO).
The morphometric study showed that significant
differences in the average density of spermatogenic
Fig. 5 Seminiferous tubules of intact immature rat testes
(a) and after 30-min exposure in the CG (b), CG ? DMSO
(c), FG (d), FG ? DMSO (e). Hematoxylin and eosin staining.
R cell retraction, N nuclei condensation, D cell detachment,
G formation of gaps, 1 regions covered by karyolysis, 2 swollen
and hyperchromic nuclei
123
Cell Tissue Bank
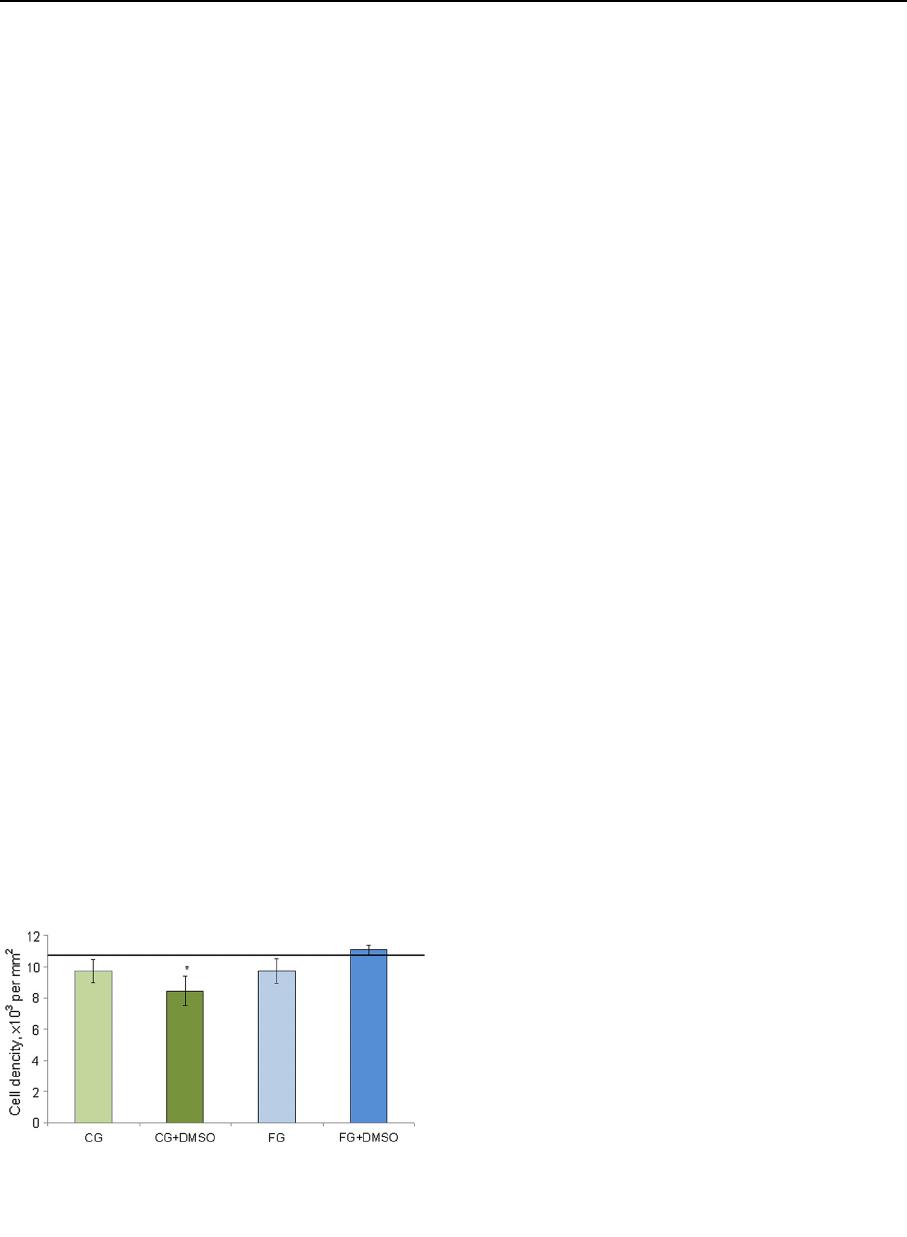
cells were absent in both investigated media without
cryoprotectant compared to the intact control (Fig. 6).
In the case of exposure of testicular tissue to DMSO
based on CG this parameter was in 1.3 times lower
than in the intact. However, this statement should be
treated with caution since according to the data
mentioned above some of the cells could have signs
of karyopicnosis/kariolysis. The use of FG as a base of
medium for cryopres ervation was more effective in
contrast to CG. In this case (FG ? DMSO), the
average cell density did not differ significantly from to
the intact control.
Discussion
Now it is absolutely clear that progress in the field of
biotechnology is inextricably linked with the devel-
opment of cryobiological approaches because low
temperature preservation allows storing biomaterial
for future medical procedures. Furthermore, cryop-
reservation makes the use of cell and tissue products
easy in time and place. Therefore, materials for such
purposes should be acceptable and even effective in
the process of freezing–thawing. This work is the first
stage in the study of the possibility to use the
biopolymer gels for improving of the efficiency of
immature testicular tissue cryopreservation that is so
necessary for prepubertal boys undergoing gonado-
toxic therapy.
Modern development of innovative biomaterials
has made a significant contribution to the development
of reproductive medicine, especially in the aspect of
maintaining fertility. At the moment hydrogels are
considered as a unique solution for stem cell storage
and transport. Their effectiveness has been studied in
relation to embryonic stem cells (Ji et al. 2004),
multipotent stem cells from bone marrow (Chen et al.
2013), adipose tissue (Swioklo et al. 2016) etc.
Nevertheless, the using of gels for tissue cryopreser-
vation is insufficiently studied. Some recent studies
(Hatte et al. 2016) have shown that DMSO with
polysaccharide-based hydrogel can be recommend ed
as a new effective tool for improving procedure of cell
or tissue low temperature preservation. The authors
note that this combination allow to ensure safety
discarding serum and to reduce DMSO concentration
and its cytotoxicity or clinical side-effects. Other
results (Itoh et al. 2001) indicate that covering with
CG improved the recovery and viability of the small
preantral fol licles during vitrification steps. Further-
more, the vitrified follicles embedded in CG could be
maintained in culture during 1 week. Microencapsu-
lation in ultrahigh viscous alginate allows maintaining
of neurosphere morphology, membrane integrity of
cells, their metabolic activity, and cell-specific inter-
actions, which are major requirements for their
applications after thawing. The use of gels in cryop-
reservation has also been studied in relation to
hepatocyte spheroids (Lee et al. 2004) and multicel-
lular tumor spheroids (Sakai et al. 2012) and opti-
mistic results were received. In the same way FG
provides optimal support for adhesion, proliferation,
differentiation and biochemical signaling of cells. It is
used to encapsulate fragments of integral tissue or
isolated cells to support their 3D structure, as well as to
study biological phenomena that would be impossible
in 2D systems (Chiti et al. 2017). The studies,
conducted by Murdock et al. (2018) showed that
extracellular matrix can promotes mitogenesis, migra-
tion, and/or differentiation of various stem/progenitor
cells and contribute to the survival of human sper-
matogonial stem cells in culture.
So encapsulation is considered at this point as a
promising approach for low temperature preservation
of 3D cell systems, as this approach protected cells
against mechani cal damages during ice crystal forma-
tion and reduce the risk of cell–cell disruption through
immobilization within the hydrogel (Malpique et al.
2010).
In this research, we examined the potency of the
using of collagen and fibrin gels as the basis of the
cryoprotective medium at the exposure stage. Colla-
gen and fibrin gels are commonly used in tissue
Fig. 6 The average density of spermatogenic epithelium cells
after 30-min exposure in the investigated media. The solid line is
an index of intact control. *The difference is statistically
significant relative to intact control (M ± m; n = 5; p \ 0.05)
123
Cell Tissue Bank

engineering as the foundational matrix due to their
availability, scaffolding function, and bioactive qual-
ities. Undoubted advantage of them is an ability to
closely mimic native extracellular matrix of tissues.
Scaffolds on basis of this substance in various forms
have been studied and employed for different tissue
regeneration purposes (Geckil et al. 2010).
It was found that FG led to the prolongation of the
exposure time of the seminiferous tubules to 4 5 min
according to the results of metabolic tests as well as to
less histological changes in the tubular structure with
preservation of the average cell density of the
spermatogenic epithelium. More pronounced protec-
tive effect of FG in our opinion is due to the presence
of different biologically active substances in its
composition in contrast to CG, whose composition is
poorer. It is known that various blood products contain
a number of growth factors (hepatocyte growth factor
(HGF), transforming growth factor b (TGF-b), plate-
let-derived growth factor (PDGF), insulin like growth
factor 1 (IGF-1), vascular endothelial growth factor
(VEGF), epidermal growth factor (EGF), nerve
growth factor (NGF) etc.) but in different ratio
(Nishiyama et al. 2016). Growth factors play a huge
role in the cell functioning and can affect cell
metabolic processes including on the stages of low
temperature preservation. Thus a significant improve-
ment was observed in viability, motility and apoptosis
level of human asthenozoospermic sperm after addi-
tion of NGF to the cryoprotective media (Saeednia
et al. 2016). The using of IGF-I reduced the ratio of
sperm with disrupted membranes and the number of
Annexin V? sperm after hypothermic storage
(Makarevich et al. 2014). VEGF treatment can prevent
germ cell death in bovine testis tissue explants due to
stimulation of an intracellular response (Caires et al.
2009). Moreover in the case of application of cryop-
reserved testicular tissue in vivo VEGF can support
engraftment of the transplant promoting angiogenesis
in the tissue (Tian et al. 2016; Del Vento et al. 2018).
Thus the use of FG as an integr al component of
freezing medium is effective and contributes to the
preservation of the average density of germinative cell
and their metabolic activity during the exposure stage
of cryopreservation. We recommend an optimal
exposure time of 30-min in the case of cryoprotective
media on the bases of CG or FG. However, 45-min
exposure of testicular tissue to FG with 0.6 M DMSO
did not impair the spermatogenic epithelium and the
metabolic activity of the cells (MTT test and LDH
activity) and therefore time interval can be increased
for this gel. In the future, we plan to study the
morphological and functional parameters of immature
testicular tissue after freezing- thawing and evaluate
the contribution of biopolymer gels to the protection of
the spermatogenic epithelium during low-temperature
storage.
Funding This study was carried-out within the research
project of the National Academy of Sciences of Ukraine (No.
2.2.6.99).
Compliance with ethical standards
Conflict of interest All authors declare that they have no
conflict of interest.
Ethical approval All the manipulations with animals were
carried out in accordance to the European convention for the
protection of vertebrate animals used for experimental and other
scientific purposes (Strasbourg, 18.III.1986). The protocols
were approved by the Bioethics Committee of Institute for
Problems of Cryobiology and Cryomedicine of the National
Academy of Sciences of Ukraine (Permit No 2016-05).
Human and animal rights This article does not contain any
studies with human participants performed by any of the
authors.
References
Abrishami M, Anzar M, Yanga Y, Honaramooz A (2010)
Cryopreservation of immature porcine testis tissue to
maintain its developmental potential after xenografting
into recipient mice. Theriogenology 73(1):86–96
Allenspach AL, Kraemer TG (1989) Ice crystal patterns in
artificialgels of extracellular matrix molecules after quick-
freezing and freeze-substitution. Cryobiology 26:170–179
Bret DU, Lakshmi SN, Cato TL (2011) Biomedical applications
of biodegradable polymers. J Polym Sci B Polym Phys
49(12):832–864
Caires KC, de Avila J, McLean DJ (2009) Vascular endothelial
growth factor regulates germ cell survival during estab-
lishment of spermatogenesis in the bovine testis. Repro-
duction 138(4):667–677
Chandrakasan G, Torchia DA, Piez KA (1976) Preparation of
intact monomeric collagen from rat tail tendon and skin
and the structure of the nonhelical ends in solution. J Biol
Chem 251:6062–6067
Chen B, Wright B, Sahoo R, Connon CJ (2013) A novel alter-
native to cryopreservation for the short-term storage of
stem cells for use in cell therapy using alginate encapsu-
lation. Tissue Eng Part C Methods 19(7):568–576
Chiti MC, Dolmans MM, Donnez J, Amorim CA (2017) Fibrin
in reproductive tissue engineering: a review on its
123
Cell Tissue Bank

application as a biomaterial for fertility preservation. Ann
Biomed Eng 45(7):1650–1663
Del Vento F, Vermeulen M, de Michele F, Giudice MG, Poels J,
des Rieux A, Wyns C (2018) Tissue engineering to
improve immature testicular tissue and cell transplantation
outcomes: one step closer to fertility restoration for pre-
pubertal boys exposed to gonadotoxic treatments. Int J Mol
Sci 19(1):E286
Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS (2011)
Polymeric scaffolds in tissue engineering application: a
review. Int J Polym Sci 2011:1–19
Geckil H, Xu F, Zhang X, Moon S, Demirci U (2010) Engi-
neering hydrogels as extracellular matrix mimics. Nano-
medicine 5(3):469–484
Gelse K, Po
¨
sch E, Aigner T (2003) Collagens—structure,
function, and biosynthesis. Adv Drug Deliv Rev
55(12):1531–1546
Guarino V, Ambrosio L (2014) Properties of biomedical foams
for tissue engineering applications. In: Netti PA (ed)
biomedical foams for tissue engineering applications.
Woodhead Publishing, Cambridge, pp 40–70
Hatte L, Le Corre S, Baudot A, Louis G, Letourneur D, Doucet
C, Meddahi-Pelle
´
A (2016) New application for biohy-
drogels: myoblast cryopreservation for cell therapy. In:
Frontiers in bioengineering and biotechnology conference
abstract: 10th world biomaterials congress. https://doi.org/
10.3389/conf.FBIOE.2016.01.00444
Itoh T, Kacchi M, Abe H, Hoshi H (2001) High recovery from
successful cryopreservation of bovine small preantral fol-
licles embedded within collagen gels. Tissue Cult Res
Commun 21:109–119
Jafari M, Paknejad Z, Rad MR, Motamedian SR, Eghbal MJ,
Nadjmi N, Khojasteh A (2017) Polymeric scaffolds in
tissue engineering: a literature review. J Biomed Mater Res
B 105(2):431–459
Ji L, de Pablo JJ, Palecek SP (2004) Cryopreserva tion of
adherent human embryonic stem cells. Biotechnol Bioeng
88(3):299–312
Keros V, Hultenby K, Borgstro
¨
m B, Fridstrom M, Jahnukainen
K, Hovatta O (2007) Methods of cryopreservation of tes-
ticular tissue with viable spermatogonia in pre-pubertal
boys undergoing gonadotoxic cancer treatment. Hum
Reprod 22(5):1384–1395
Koebe HG, Dunn JC, Toner M, Sterling LM, Hubel A, Cravalho
EG, Yarmush ML, Tompkins RG (1990) A new approach
to the cryopreservation of hepatocytes in a sandwich cul-
ture configuration. Cryobiology 27(5):576–584
Lee KW, Park JB, Yoon JJ, Lee JH, Kim SY, Jung HJ, Lee SK,
Kim SJ, Lee JH, Lee DS, Joh JW (2004) The viability and
function of cryopreserved hepatocyte spheroids with dif-
ferent cryopreservation solutions. Transpl Proc
36(8):2462–2463
Li Y, Meng H, Liu Y, Lee BP (2015) Fibrin gel as an
injectable biodegradable scaffold and cell carrier for tissue
engineering. Sci World J 2015:685690
Makarevich AV, Spalekova E, Olexikova L, Kubovicova E,
Hegedusova Z (2014) Effect of insulin-like growth factor I
on functional parameters of ram cooled-stored spermato-
zoa. Zygote 22(3):305–313
Malpique R, Osorio LM, Ferreira DS, Ehrhart F, Brito C,
Zimmermann H, Alves PM (2010) Alginate encapsulation
as a novel strategy for the cryopreservation of neuro-
spheres. Tissue Eng Part C Methods 16(5):965–977
Michele F, Vermeulen M, Wyns C (2017) Fertility restoration
with spermatogonial stem cells. Curr Opin Endocrinol
24(6):424–431
Milazzo JP, Vaudreuil L, Cauliez B, Gruel E, Masse L, Mousset-
Simeon N, Mace B, Rives N (2008) Comparison of con-
ditions for cryopreservation of testicular tissue from
immature mice. Hum Reprod 23:17–28
Milazzo JP, Travers A, Bironneau A, Safsaf A, Gruel E, Arnoult
C, Mace B, Boyer O, Rives N (2010) Rapid screening of
cryopreservation protocols for murine prepubertal testicu-
lar tissue by histology and PCNA immunostaining. J An-
drol 31(6):617–630
Mossman T (1983) Rapid colorimetric assay for cellular growth
and survival: application to proliferation and cytotoxicity
assays. J Immunol Methods 65(1–2):55–63
Murdock MH, David S, Swinehart IT, Reing JE, Tran K, Gassei
K, Orwig KE, Badylak SF (2018) Human testis extracel-
lular matrix enhances human spermatogonial stem cell
survival in vitro. Tissue Eng Part A. https://doi.org/10.
1089/ten.TEA.2018.0147
Nair LS, Laurencin CT (2007) Biodegradable polymers as
biomaterials. Progr Polym Sci 32:762–798
Nicodemus GD, Bryant SJ (2008) Cell encapsulation in
biodegradable hydrogels for tissue engineering applica-
tions. Tissue Eng Part B Rewires 14:149–165
Nishiyama K, Okudera T, Watanabe T, Isobe K, Suzuki M,
Masuki H, Okudera H, Uematsu K, Nakata K, Kawase T
(2016) Basic characteristics of plasma rich in growth fac-
tors (PRGF): blood cell components and biological effects.
Clin Exp Dent Res 2(2):96–103
Saeednia S, Shabani Nashtaei M, Bahadoran H, Aleyasin A,
Amidi F (2016) Effect of nerve growth factor on sperm
quality in asthenozoosprmic men during cryopreservation.
Reprod Biol Endocrinol 14(1):29
Sakai S, Inamoto K, Liu Y, Tanaka S, Arii S, Taya M (2012)
Multicellular tumor spheroid formation in duplex micro-
capsules for analysis of chemosensitivity. Cancer Sci
103(3):m549–m554
Swioklo S, Constantinescu A, Connon CJ (2016) Alginate-en-
capsulation for the improved hypothermic preservation of
human adipose-derived stem cells. Stem Cell Transl Med
5(3):339–349
Takahashi T, Hirsh A, Erbe E, Williams RJ (1988) Mechanism
of cryoprotection by extracellular polymeric solutes. Bio-
phys J 54(3):509–518
Tian R, Yang S, Zhu Y, Zou S, Li P, Wang J, Zhu Z, Huang Y,
He Z, Li Z (2016) VEGF/VEGFR2 signaling regulates
germ cell proliferation in vitro and promotes mouse tes-
ticular regeneration in vivo. Cells Tissues Organs
201(1):1–13
Travers A, Milazzo JP, Perdrix A, Metton C, Bironneau A, Mace
B, Rives N (2011) Assessment of freezing procedures for
rat immature testicular tissue. Theriogenology
76(6):981–990
Volkova NO, Yukhta MS, Chernyshenko LG, Stepanyuk LV,
Sokol LV, Goltsev AM (2017) Exposure of seminiferous
tubules of immature rats to cryoprotective media of various
compositions. Probl Cryobiol Cryomed 27(3):203–218
123
Cell Tissue Bank
