RES E A R C H A R T I C L E Open Access
Effectiveness of lidocaine/prilocaine cream
on cardiovascular reactions from
endotracheal intubation and cough events
during recovery period of older patients
under general anesthesia: prospective,
randomized placebo-controlled study
Linsheng Lv
1†
, Yan Lei
2†
, Liu Xun
3*
and Miaoxiaiven Chen
4*
Abstract
Background: Endotracheal intubation is known to pose significant physiological, pharmacokinetic, and dynamic
changes and postoperative respiratory complications in patients under general anesthesia.
Method: An RCT trial was organized by the Third Affiliated Hospital at Sun Yat-sen University, China. Patients were
eligible for inclusion in the trial if they were over 60 years old and had upper-abdominal surgery during the
induction of anesthesia and had enrolled in endotracheal intubations. The primary end point included
cardiovascular reactions during the induction of anesthesia and endotracheal intubations and cough events during
the recovery period. In the test group, 2 g of lidocaine/prilocaine cream (and in the control group, 2 g of Vaseline)
were laid over the surface of the tracheal tube cuff.
Results: The systolic blood pressure (F value = 62.271, p < 0.001), diastolic blood pressure (F value = 150.875, p <
0.001), and heart rate (F value = 75.627, p < 0.001) of the test group were significantly lower than the control group.
Cough events during the recovery period in the test group were better (spontaneous cough, χ
2
value = 10.591, p <
0.001; induced cough, χ
2
value =10.806 , p < 0.001).
Conclusion: In older patients, coughing and cardiovascular reactions under anesthesia and endotracheal
intubations were reduced, as a result of using lidocaine/prilocaine cream on the surface of the tracheal tube cuff.
Trial registration: International Clinical Trials Network NCT02017392, 2013-12-16.
© The Author(s). 2020 Open Access This article is licensed under a Creative Commons Attribution 4.0 Intern ational License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if
changes were made. The images or other third party material in this article are included in the article's Creative Commons
licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the
data made available in this article, unless otherwise stated in a credit line to the data.
†
Lv Linsheng and Yan Lei contributed equally to this work.
3
Division of Nephrology, the third Affiliated Hospital of Sun Yat-sen
University, Guangzhou 510630, Guangdong, China
4
Nursing Department, the third Affiliated Hospital of Sun Yat-sen University,
Guangzhou 510630, Guangdong, China
Full list of author information is available at the end of the article
Lv et al. BMC Geriatrics (2020) 20:157
https://doi.org/10.1186/s12877-020-01567-y
Background
Endotracheal intubation has been shown to cause signifi-
cant physiological, pharmacokinetic, and dynamic changes,
along with postoperative respiratory complications in pa-
tients under general anesthesia [1]. Transient hemodynamic
changes during these periods increase myocardial oxygen
consumption, leading to myocardial ischemia. This can in-
crease the risk of cardiovascular and cerebrovascular dis-
eases, and of morbidity and mortality, in older patients
under general anesthesia. Stellate ganglion block can re-
strain the stress response from inducing anesthesia and
endotracheal intubations [2]. Several alternative approaches
to mitigating this response have also been reported, such as
the administr ation of esmolol, labetalol, nitroglycerin, cloni-
dineare, and lidocaine [3–7]. However, stellate ganglion
blocks are invasive and the alternative approaches are not
always satisfactory.
Lidocaine/prilocaine cream (a eutectic mixture of lido-
caine 25 mg/mL and prilocaine 25 mg/mL) is widely
used as a topical anesthetic [ 8–11]. To our knowledge,
however, no high quality randomized con trolled study
has been conducted to investigate the use of lidocaine/
prilocaine cream to prevent cardiovascular stress re-
sponses in older patients undergoing tracheal intubation
under general anesthesia. Our central hypothesis is that
lidocaine/prilocaine can be used substantially in tracheal
intubation of older patients under general anesthesia.
The purpose of our study was to carry out an RCT trial
on this application. The results offer accurate evidence
for the local use of lidocaine/prilocaine cream in tracheal
intubation of older patients under general anesthesia, to
facilitate the postoperative rehabilitation and quality of
life of these patients.
Methods
Trial design
The RCT trial was organized by the Third Affiliated
Hospital at Sun Yat-sen University, China. The trial was
conducted according to the CONSORT-2010 guidelines.
The study was approved by the Review Board of the
Third Affilia ted Hospital at Sun Yet-sen University
([2013]2–85). All subjects provided written informed
consent before the trial. Underlying data are available in
accordance with the Management of Human Genetic
Resources in China.
Participants and setting
Patients were included in the trial if they were over 60
years old and scheduled for upper abdominal surgery
under general anesthesia using endotracheal tube intub-
ation from August 2015 through December 2018. The
major exclusion criteria were the following: allergies to
lidocaine, prilocaine, or any other ingredients in the test
product; ischemic heart disease or advanced chronic
kidney disease; younger than 60 years old; American
Society of Anesthesiologists (ASA ) Grade IV. We ran-
domly assigned old er patients t o either lidoca ine/prilo-
caine cream treatment or a placebo (Vaseline) at a 1:1
ratio. The primary end point included cardiovascular
reactions during the induction of a nesthesia, along with
endotracheal intubations a nd cough e vents during the
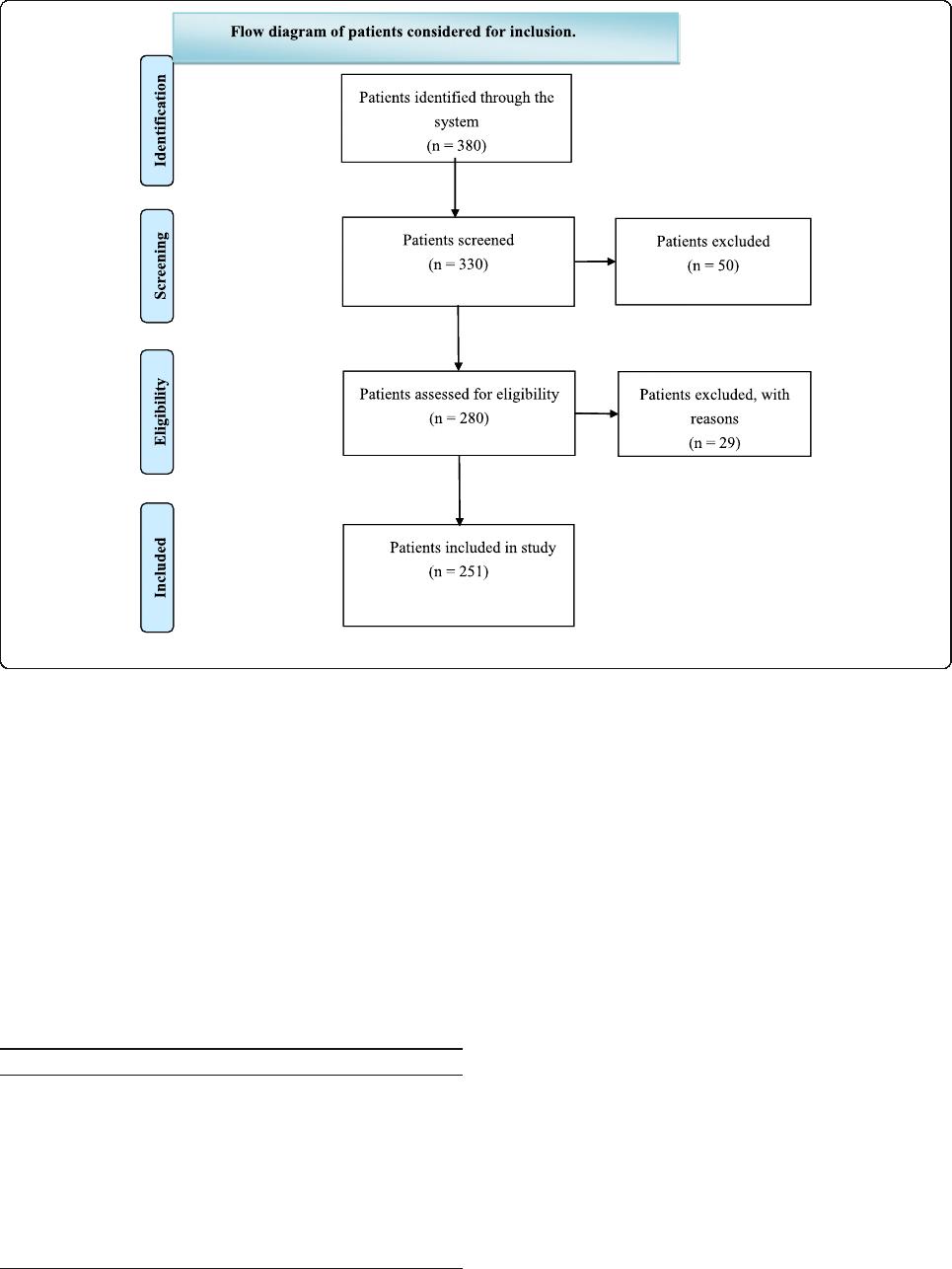
recovery per iod. Figure 1 shows a flowchart for the as-
signment of participants in the study.
Intervention
Nurses inflated the endotracheal tube. According to the
random number table, in the test group, off-label use of
2 g of lidocaine/prilocaine cream (and in the control
group, 2 g of Vaseline cream) were laid over the surface
of the tracheal tube cuff. After ventilation, the anesthesi-
ologists placed the endotracheal tube and fixed the
endotracheal tube with air. Both the patients and the in-
vestigators were unaware of the trial-group assignments.
Before tracheal intubation, anesthetics was used and un-
conscious intub ation was carried out. The depth of
anesthesia was measured by the bispectral index (BIS).
Fentanyl was used as the induction agent according to
the sex, weight, and ASA rating of the patients. Benzene
sulfuro acted as the muscle relaxant and its dosage was
based on the weight and situation of the patients during
the operation.
Parameter measurement
Cardiovascular reaction during the induction of anesthesia:
systolic blood pressure (SBP) (mm Hg), diastolic blood
pressure (DBP) (mm Hg), heart rate (HR) (beats/min), and
cough reaction. Coughing that occurred during extubation
was defined as induced cough. The SBP, DBP, and HR were
measured with a monitor.
Sample size calculation
According to the pret est analysis of 10 patients in each
group, we set α = 0.05 and β = 0.20, with a sample drop
rate of 20%. Using Open Epi Version 2 [12 ], we calcu-
lated the minimum sample size of 125 cases in each
group (a total of 250 cases).
Statistical analysis
The measurements were calcu lated as the mean (±
standard deviation). An independent t-test was used to
compare the groups. Further, a chi-squared test was
used to compare the categorized data in the groups. An
analysis of variance of the repeated measurement data
was conducted to compare the data. We set p < 0.05 as
statistically significant. R version 3.0.2 was used to
process the data.
Lv et al. BMC Geriatrics (2020) 20:157 Page 2 of 5
Results
Patients
A total of 251 older patients at the Third Affiliated
Hospital at Sun Yet-sen University were enrolled in
the randomized trial. There were no significant differ-
ences in the baseline characteristics (such as age, sex,
ASA rating, operation time and depth of anesthesia)
of the patients between the two groups (Table 1).
Both groups were anesthetized with general anesthesia
by tracheal intubation.
Primary outcomes
The systolic blood pressure (F value = 62.271, p < 0.001),
DBP (F value = 150.875, P < 0.001) and heart rate (F
value = 75.627, P = < 0.001) of the test group were signifi-
cantly lower than the control group (Table 2).
Cough events in recovery period in the test group were
better (Spontaneous cough (χ
2
value = 10.591, P = 0.001);
Induces cough (χ
2
value =10.806, P = 0.001)) (Table 3).
Discussion
To our kno wledge, this study for the first time demon-
strated in a high quality RCT that there are improve-
ments from the use of lidocaine/prilocaine cream on the
surface of the tracheal tube cuff in old er patients in
terms of postoperative coughing and cardiovascular re-
actions during the induction of anesthesia. Our results
are similar to those by Chen [2]. Furthermore, our study
found that cough events during the recovery period im-
proved in the test group. These findings may lead to
beneficial effects on cardiovascular reactions in the
course of endotracheal intubation.
During tracheal intubation, blood pressure often rises
sharply, and systolic blood pressure rises by 45 mmHg
Fig. 1 Flow diagram of patients considered for inclusion.
Table 1 Patient Characteristics
Test Group Control Group t/χ
2
Value P Value
Age (years) 70.3 ± 5.9 70.4 ± 5.2 −0.155 0.877
Sex (male n (%)) 76 (53.9) 65 (46.1) 2.163 0.141
ASA I (%) 44 (51.8) 41 (48.2) 0.752 0.687
ASA II(%) 38 (52.1) 35 (47.9)
ASA III(%) 43 (46.2) 50 (53.8)
Operation time (hours) 1.6 ± 0.6 1.6 ± 0.6 t = −0.693 0.489
Depth of anaesthesia 48.3 ± 4.4 47.6 ± 4.5 t = 1.279 0.202
Lv et al. BMC Geriatrics (2020) 20:157 Page 3 of 5

on average. Further, tachycardia and other circulatory
system reactions are common, and they are collectively
referred to as the intubation stress response. Generally,
the time is short (3–5 min). However, patients with an
abnormal cardiovascular and cerebrovascular system, es-
pecially older patients, face life-threatening reactions
that should be minimized or avoided completely [13].
Lidocaine and prilocaine are phthalocaine local anes-
thetics. Lidocaine is fast-acting, with wide dispersion,
strong penetration, and no obvious vasodilator effect.
The structure of prilocaine is similar to that of lidocaine
and it decomposes easily, such that its toxicity is rela-
tively rare. Its onset time is slower than that of lidocaine,
and its duration is slightly longer. The combined appli-
cation of the two drugs can enhance the anes thetic ef-
fect. Moreover, it takes effect quickly with a long
duration. It has antimicrobial properties of intact human
skin flora as a topical anesthesia before vascular access,
and reduces the pain of venipuncture in hemodialysis
patients [8–11 ].
The strength of our paper lies in its high-quality de-
sign. However, there are limitations: 1) the total observa-
tion period was relatively short, and indeed a longer
observation time may be better; 2) although our treat-
ment was very safe, we did not extensively analyze side
effects; 3) we did not set up clinically relevant cutoffs re-
garding blood pressure; 4) we did not study significant
postoperative symptoms such as postoperative sore
throat or difficulty swallowing in this paper; and 5) the
coughing intensity was not measured.
Conclusion
We found that during the induction of anesthesia in
older patients, cough reactions and cardiovascular
reactions from endotracheal intubations improved as a
result of using lidocaine/prilocaine cream on the surface
of the tracheal tube cuff, which may decrease the risk of
cardiovascular and cerebrovascular diseases in these
patients.
Abbreviations
RCT: Randomized controlled study; SBP: Systolic blood pressure;
DBP: Diastolic blood pressure; HR: Heart rate
Acknowledgements
We would like to thank all the doctors, nurses, technicians, and patients
involved in this study for their cooperation.
The authors thank Dr. David Cushley from International Science Editing,
Clare, Ireland for assistance with the English version of the manuscript.
Authors’ contributions
Author Contributions: LLS and CMX researched data. LLS, YL and LX wrote
manuscript. LX and CMX reviewed/edited manuscript. LX and CMX
contributed to discussion. The author(s) read and approved the final
manuscript.
Funding
Project 81370866 supported by the National Natural Science Foundation of
China in the design of the study, interpretation of data, and in writing the
manuscript.
Availability of data and materials
The database used and/or analyzed during the current study are available
from the corresponding author (Prof. Xun Liu, Division of Nephrology, the
third Affiliated Hospital of Sun Yat-sen University, Guangzhou 510630, China.
Ethics approval and consent to participate
The study was approved by the Review Board of the Third Affiliated Hospital
at Sun Yet-sen University ([2013]2–85). All subjects provided written informed
consent before the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Author details
1
Operation Room, the third Affiliated Hospital of Sun Yat-sen University,
Guangzhou 510630, Guangdong, China.
2
Shanghai Shyndec Pharmaceutical
Co., Ltd, Shanghai 600420, China.
3
Division of Nephrology, the third Affiliated
Hospital of Sun Yat-sen University, Guangzhou 510630, Guangdong, China.
4
Nursing Department, the third Affiliated Hospital of Sun Yat-sen University,
Guangzhou 510630, Guangdong, China.
Table 2 Comparison of cardiovascular reaction and heart rate between two groups
Group Before
induction
Before
intubation
Instant
intubation
1 min after
intubation
Instant
extubation
1 min after
extubation
3 min after
extubation
F Value P Value
SBP (mm Hg) Test group 141.8 ± 5.9 131.8 ± 5.3 128.8 ± 4.8 126.8 ± 4.6 123.8 ± 3.9 120.8 ± 3.7 119.9 ± 3.4 62.271 < 0.0001
Control group 139.7 ± 5.4 133.8 ± 4.9 131.8 ± 4.6 130.8 ± 4.2 131.9 ± 3.7 129.8 ± 3.5 127.9 ± 3.3
DBP (mm Hg) Test group 81.8 ± 5.9 74.8 ± 5.3 73.8 ± 4.8 74.8 ± 4.6 69.8 ± 3.9 71.8 ± 3.7 69.9 ± 3.4 150.875 < 0.0001
Control group 82.7 ± 5.4 75.8 ± 4.9 81.8 ± 4.6 80.8 ± 4.2 82.9 ± 3.7 80.8 ± 3.5 79.9 ± 3.3
HR (beat/min) Test group 85.8 ± 5.9 77.8 ± 5.3 82.8 ± 4.8 80.8 ± 4.6 82.8 ± 3.9 80.8 ± 3.7 82.9 ± 3.4 75.627 < 0.0001
Control group 85.7 ± 5.4 77.8 ± 4.9 89.8 ± 4.6 88.8 ± 4.2 89.9 ± 3.7 88.8 ± 3.5 86.9 ± 3.3
a
SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate; min: minute
Table 3 Comparison of cough events during recovery period
between two groups
Treatment
Group
Control
Group
χ
2
Value P Value
Spontaneous cough [case (%)] 4 (3.1) 13 (10.7) 10.591 0.001
Induces cough [case (%)] 8 (6.4) 27 (21.4) 10.806 0.001
Lv et al. BMC Geriatrics (2020) 20:157 Page 4 of 5

Received: 28 February 2020 Accepted: 22 April 2020
References
1. Beyer K, Taffé P, Halfon P, Pittet V, Pichard S, Haller G, Burnand B, ADS study
group. Hypertension and intra-operative incidents: a multicentre study of
125,000 surgical procedures in Swiss hospitals. Anaesthesia. 2009;64(5):494–502.
2. Chen YQ, Jin XJ, Liu ZF, Zhu MF. Effects of stellate ganglion block on
cardiovascular reaction and heart rate variability in elderly patients during
anesthesia induction and endotracheal intubation. J Clin Anesth. 2015;27(2):
140–5.
3. Chung KS, Sinatra RS, Chung JH. The effect of an intermediate dose of
labetalol on heart rate and blood pressure responses to laryngoscopy and
intubation. J Clin Anesth. 1992;4(1):11–5.
4. Zalunardo MP, Zollinger A, Szelloe P, Spahn DR, Seifert B, Pasch T.
Cardiovascular stress protection following anesthesia induction. Comparison
of clonidine and esmolol. Anaesthesist. 2001;50(1):21–5.
5. Firoozbakhsh F, Mohammadi FH, Safari S, Khashayar P. The effect of
intravenous nitroglycerine on blood pressure during intubation. Middle East
J Anesthesiol. 2008;19(4):859–67.
6. Menigaux C, Guignard B, Adam F, Sessler DI, Joly V, Chauvin M. Esmolol
prevents movement and attenuates the BIS response to orotracheal
intubation. Br J Anaesth. 2002;89(6):857–62.
7. Sun HL, Wu TJ, Ng CC, Chien CC, Huang CC, Chie WC. Efficacy of
oropharyngeal lidocaine instillation on hemodynamic responses to
orotracheal intubation. J Clin Anesth. 2009;21(2):103–7.
8. Batai I, Bogar L, Juhasz V, Batai R, Kerenyi M. A comparison of the
antimicrobial property of lidocaine/prilocaine cream (EMLA) and an alcohol-
based disinfectant on intact human skin flora. Anesth Analg. 2009;108(2):
666–8.
9. Sawyer J, Febbraro S, Masud S, Ashburn MA, Campbell JC. Heated lidocaine/
tetracaine patch (Synera, Rapydan) compared with lidocaine/prilocaine
cream (EMLA) for topical anaesthesia before vascular access. Br J Anaesth.
2009;102(2):210–5.
10. Çelik G, Özbek O, Yılmaz M, Duman I, Özbek S, Apiliogullari S. Vapocoolant
spray vs lidocaine/prilocaine cream for reducing the pain of venipuncture in
hemodialysis patients: a randomized, placebo-controlled, crossover study.
Int J Med Sci. 2011;8(7):623–7.
11. Keiji F, Hiroki A, Yasuo I, Hitoshi Y. Comparison of the pain-reducing effects
of EMLA cream and of lidocaine tape during arteriovenous fistula puncture
in patientsundergoing hemodialysis: A multi-center, open-label, randomized
crossover trial. PLoS One. 2020;15(3):e0230372.
12. Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic
Statistics for Public Health, www.OpenEpi.com, updated 2013/04/06.
Accessed 26 Apr 2020.
13. Ko BJ, Oh JN, Lee JH, Choi SR, Lee SC, Chung CJ. Comparison of effects of
fentanyl and remifentanil on hemodynamic response to
endotrachealintubation and myoclonus in elderly patients with etomidate
induction. Korean J Anesthesiol. 2013;64(1):12–8.
Publisher’sNote
Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations.
Lv et al. BMC Geriatrics (2020) 20:157 Page 5 of 5
