
Structure and Viscosity of Molten CaO-SiO
2
-Fe
x
O
Slag During the Early Period of Basic Oxygen
Steelmaking
RUI ZHANG, YU WANG, XUAN ZHAO, JIXIAN G JIA, CHENGJUN LIU, and YI MIN
According to the composition variation during the initial period of basic oxygen steelmaking,
ice-quenched samples of the CaO-SiO
2
-Fe
x
O system were prepared, and the viscosity and
structure of molten slag were further analyzed by a rotary viscometer and Raman spectroscopy,
respectively. The results showed that Si
4+
existed as Q
0
,Q
1
,Q
2
and Q
3
units. The O
2
ions led
to the depolymerization of [SiO
4
] tetrahedrons from Q
3
to Q
0
units with increasing Ca/Fe ratio.
For Fe
3+
cations, two types of [FeO
4
] tetrahedron and [FeO
6
] octahedron coexisted in the
molten slag, and coordination of Fe
3+
transformed from tetrahedron to octahedron with the
Ca/Fe ratio increasing to 3.18. Viscosity of molten slag showed a continuous decrease because
of the simpler network. Moreover, to clarify the viscosity-structure relationship, the viscos ity
estimation equation applied to the CaO-SiO
2
-Fe
x
O-based system was established in terms of the
deconvolution result of the melt structure.
https://doi.org/10.1007/s11663-020-01888-8
The Minerals, Metals & Materials Society and ASM International 2020
I. INTRODUCTION
BASIC oxygen steelmaking is widely used in the
primary smelting process of steel because of the better
smelting effect and the shorter smelting cycle. The core
task of basic oxygen steelmaking is to control the
appropriate chemical composition and temperature of
molten steel by desiliconization, dephosphorization and
decarburization. However, the different smelting periods
have different functions during basic oxygen steelmak-
ing. The main task of the initial smelting period is to
promote the dephosphorization reaction by rapid slag-
ging. At this period, molten slag plays an irreplaceable
metallurgical role in fixing phosphorus transferred from
molten steel and controlling the slag-steel reaction rate.
These metallurgical functions are closel y related to the
viscosity of the converter slag. The suitable viscous flow
of molten slag can facilitate the chemical reaction to
eliminate impurity elements and control the heat trans-
fer, mass transfer and smelting stability related to the
active multiphase reaction among molten slag, liqui d
steel and gas.
[1,2]
To adjust the viscosity of molten slag, the relationship
of the viscosity-structure-composition of converter slag
was investigated in previous studies,
[3–7]
which showed
that the increasing CaO/SiO
2
ratio and MgO resulted in
lower viscosity of the converter slag because of a
decrease in the complex structures of the [SiO
4
] tetra-
hedron. The structural role of MgO is similar to that of
CaO, which could cut off the bridging oxygen of the
[SiO
4
] tetrahedron. Therefore, investigations on the
structures of molten slag are essential to gain a thorough
comprehension of the viscosity of metallurgical slag
systems.
Considering the structure of the converter slag, the
structural behaviors of silicon as a network-forming ion
are explicit, and it can form the tetrahedral unit by
combining oxygen to construct a three-dimensional
network structure.
[8]
The structural behaviors of alkali
and alkaline earth metals in converter slag are also clear.
For example, as typical network modifiers, calcia and
magnesia can dissociate into Ca
2+
,Mg
2+
and O
2 [9]
The dissociated O
2
can cut off the bridging oxygen
bonds of the network structure, forming two non-bridg-
ing oxygen bonds. The dissociated Ca
2+
and Mg
2+
,
called compensator cations, play a role in compensating
the electronegativity of complex anion groups such as
RUI ZHANG, YU WANG, XUAN ZHAO, and CHENGJUN
LIU are with the Key Laboratory for Ecological Metallurgy of
Multimetallic Ores (Ministry of Education), Shenyang 110819,
Liaoning, P.R. China, and also with the School of Metallurgy,
Northeastern University, Shenyang 110819, Liaoning, P.R. China.
JIXIANG JIA is with the State Key Laboratory of Metal Material for
Marine Equipment and Application, Anshan 114021, Liaoning, P.R.
China. YI MIN is with the Key Laboratory for Ecological Metallurgy
of Multimetallic Ores (Ministry of Education), Shenyang 110819,
Liaoning, P.R. China, and also with the School of Metallurgy,
Northeastern University, Shenyang 110819, Liaoning, P.R. China, and
also with the Key Laboratory for Ecological Metallurgy of
Multimetallic Ores (Ministry of Education), Shenyang 110819,
Manuscript submitted July 31, 2019.
METALLURGICAL AND MATERIALS TRANSACTIONS B
the SiO
4
unit to maintain the electrical neutrality of the
network. However, other important components of
converter slag have more complicated struc tural behav-
iors. For example, the valence values of iron ions are not
fixed. Even though the valence values of iron ions are
identical, different structural types are formed by iron
ions. Mysen
[10]
and Virgo
[11]
thought that Fe
3+
cations
behaved as network formers as well as network mod-
ifiers and Fe
2+
cations only existed as network modifiers
in the SiO
2
-Al
2
O
3
-Fe
2
O
3
-based slag. Zhang et al.
[12]
also
reported that the larger concentration of Fe
3+
cations
resulted in the coexis tence of a [FeO
4
] tetrahedron and
[FeO
6
] octahedron. In addition, some studies
[13,14]
revealed that Fe
2+
cations were chiefly regarded as
network formers in the K
2
O-FeO-SiO
2
systems, and
only when divalent cations were absent would a few
ferrous ions fill the larger gaps caused by ferrous ions
because of Fe
3+
merging into the sites of silicate
tetrahedrons. Consequently, there is no consensus on
the structures of converter slag containing Fe
2+
and
Fe
3+
. Particularly research on the evolution of struc-
tural units of Fe
3+
in converter slag is relatively lacking.
Currently, although the initial period of basic oxygen
steelmaking is considered the fundamental stage of
acquiring satisfactory liquid steel, the slagging process in
this stage has not been paid attention yet. In the present
work, focusing on the evolution of the slag composition
during the initial period of the basic oxygen steelmaking
process, the viscosity and structure of the simplified
CaO-SiO
2
-Fe
x
O slag system are examined, which can
reveal the viscosity transition from the perspective of the
melt structure. Moreover, the quantitative relationship
between the viscosity and melt structure is established in
terms of the deconvolution results of Raman spectra.
The results contribute to understanding the macroscopic
properties and control metallurgical behavior of con-
verter slag and thereby provide theoretical guidance for
designing the slagging route for basic oxygen
steelmaking.
II. EXPERIMENTAL PROCEDURES
A. Design of Converter Slag
For traditional basic oxygen steelmaking, composi-
tions of converter slag mainly consist of CaO, SiO
2
and
Fe
x
O, with a small amount of MgO, MnO and P
2
O
5
.
Considering the chemical property and structural role,
MgO is similar to CaO and MnO is similar to FeO.
[15]
The influence of P
2
O
5
on the melt structure is not
considered because there is relatively less content in the
molten slag. As a result, the CaO-SiO
2
-Fe
x
O ternary
system is designed as the experimental slag system in
present study.
During the initial smelting period, silicon and iron
elements in the converter slag are preferentia lly oxidized
to silica and iron ox ide. Then, the generated silica and
iron oxide could penetrate into the interior slag layer
along the capillary of lime (the main component of lime
is CaO), promoting the melting of lime. In this reaction
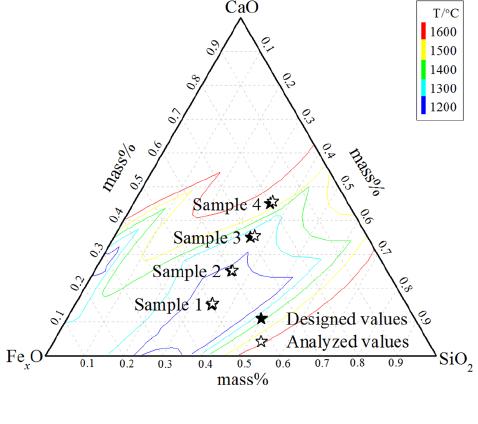
process, the CaO content increases from 17 to 32
mass pct, the FeO content decreases from 30 to 15
mass pct, the total iron content also decreases from 28
to 17 mass pct, and SiO
2
content stabilizes ne ar 20
mass pct.
[16,17]
Referring to these data, the specific
composition of the designed slag is plotted in Figure 1.
B. Pre-melted Slag Preparation
All the slag samples in the experiments were prepared
using analytical reagent grade CaO, SiO
2
and
Fe
2
C
2
O
4
Æ2H
2
O. When the temperature exceeds
1123 K, FeO can be obtained from the composi tion of
Fe
2
C
2
O
4
Æ2H
2
O.
[18]
CaO and SiO
2
were calcined at
1273 K for 4 h to remove moisture and volatile impu-
rities. The Fe
2
C
2
O
4
Æ2H
2
O powder was calcined at 873 K
for 4 h. Samples were loaded in a platinum crucible and
suspended with Mo wire in the constant temperature
zone of a high-temperature quenching furnace. The
schematic representation of a high-temperature quench-
ing furnace is shown in Figure 2. Thereafter, samples
were heated up to a temperature of 1873 K and held at
that temperature for 4 h to reach homogenization.
[19]
During the preparation of the sample, a constant flow
rate of argon (0.8 LÆmin
1
, purity > 99.9999 pct) was
maintained. The oxygen partial pressure of the system
was monitored by a ZrO
2
-CaO oxygen probe produced
by Australian Oxytrol Systems Pty. Ltd. The measured
oxygen partial pressure was controlled at about 10
10
atm, in which condition FeO was considered as the
coexistence of bivalent and trivalent irons in the molten
slags. A similar result was reported by Osugi et al.
[20]
Thereafter, the crucible fell rapidly into ice water by
opening the removable cap and loosening the Mo wire;
then, the quenched samples could be obtained.
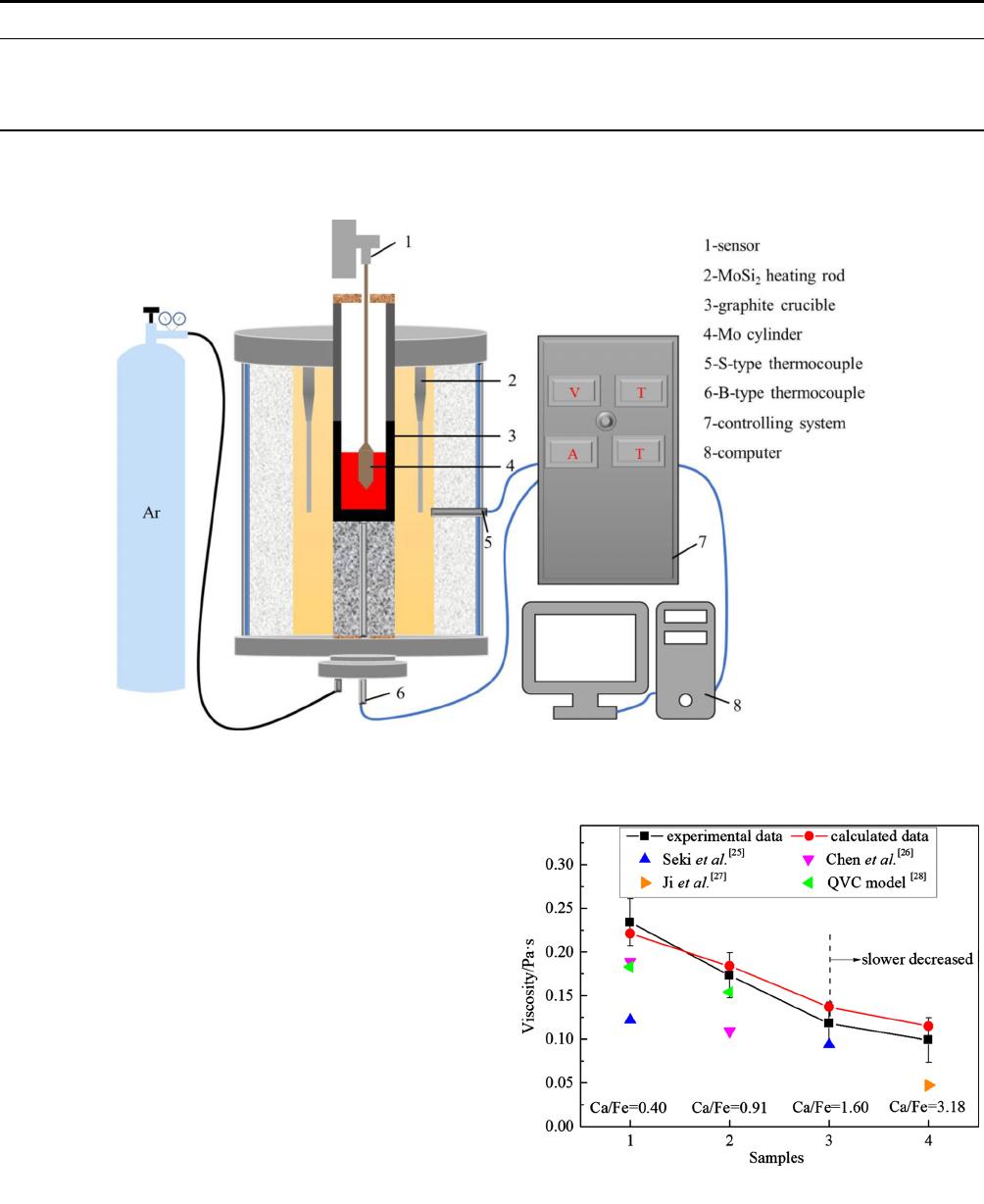
The X-ray diffraction (XRD) patte rns over the range
of 2h = 10 to 80 deg were performed using a X’pert
PRO diffractometer (PANalytiical, Holland), which
determined whether glassy samples were achieved. The
phase analyses of quenched samples are shown in
Figure 3. All the XRD profiles only showed a broad
peak around the diffraction angle 2h of 30. This
Fig. 1—Composition distribution of synthetic samples.
METALLURGICAL AND MATERIALS TRANSACTIONS B
so-called hola pattern confirmed that the samples were
amorphous.
[21]
Homogeneous glassy samples could be
considered to maintain the high-temperature state of the
melt structure and substituted the high-temperature
molten slag for analyzing the structures.
[22–24]
Additionally, compositions of glassy samples were
analyzed by an X-ray fluorescence spectrometer (S4
explorer, Germany). Yet, the iron ions in Fe
x
O could be
not specified as Fe
2+
or Fe
3+
by XRF, so the values of
Fe
2+
/
P
Fe and Fe
3+
/
P
Fe were ascertained by the
direct analysis of Fe
2+
using the K
2
Cr
2
O
7
titration
method (JIS M 8212:2005). The analyzed compositions
of glassy samples are listed in Table I and plotted in
Figure 1, which shows that the SiO
2
, CaO and total iron
contents are consistent with the designed compositions
of slag samples, and the values of Fe
3+
/Fe
2+
in glassy
samples are also close to redox ratio of iron in the
industrial slag.
[16]
C. Experimental Method
The viscosity of experimental samples was measured
by the rotating cylinder method. The experimental
apparatus for high-temperature viscosity measurements
is shown in Figure 4. About 140 g of pre-melted slag
was put into the graphite crucible and heated to 1873 K
for 30 min. The viscosity of the measured sample
remained stable with altering rotation speed, indicating
that the molten slag had been completely melted and
was Newtonian fluid. Then, the viscosity of molten slag
was measured every other minute with a Mo spindle at a
constant temperature of 1873 K. The measurement of
each sample was carried out three times. Similar
experimental methods have been described in our
previous work elsewhere.
The measurements of melt structures were carried out
using a Raman spectrometer (JY-HR800, France).
About 1 mg of slag sample was flattened and placed
on the sample stage. The Raman spectra were recorded
by a multichannel modular triple Raman system with an
excitation wavelength of 488 nm and a 1-mW semi con-
ductor laser as a light source. The measured range of the
frequency band was from 100 to 4000 cm
1
, and the
resolution of the spectrum was 0.65 cm
1
.
Fig. 2—Schematic representation of high-temperature quenching furnace.
Fig. 3—X-ray diffraction pattern of quenched samples.
METALLURGICAL AND MATERIALS TRANSACTIONS B
III. RESULTS AND DISCUSSION
A. Viscosity Analysis of Slag
The viscosity of CaO-SiO
2
-Fe
x
O slags with various
Ca/Fe ratios is shown in Figure 5. With the Ca/Fe ratio
increasing from 0.40 to 3.18, the average viscosity values
gradually decreased. A similar trend was reported by
Seki et al.
[25–28]
In addition, the viscosity of CaO-SiO
2
-
Fe
x
O slag shows a dramatic decrease at a lower Ca/Fe
ratio (0.40 to 1.60), while the viscosity decreases slowly
at a higher Ca/Fe ratio (1.60 to 3.18). Generally, the
viscous property of molten slag is affected by its
structure. For further clarification, the melt structures
of experi mental slags were also studied, and they will be
discussed in the following section.
B. Microstructural Analysis of Viscosity
All the original Raman spectra of quenched slags are
presented in Figure 6. The spectra can be divided into
three regions: the low-fr equency region (LF: 400 to
500 cm
1
), intermediate-frequency region (MF: 500 to
780 cm
1
) and high-frequency region (HF: 780 to
1200 cm
1
). Accord ing to previous studies, different
regions correspond to the various structural vibrations.
The HF region has been traditionally interpreted as the
Table I. Analysis Result of Sample Compositions (Mass Percent)
Sample Basicity CaO SiO
2
FeO Fe
2
O
3
Fe
3+
/Fe
2+
Total Iron Ca/Fe
1 0.63 15.43 35.09 32.64 16.84 0.46 37.17 0.40
2 0.87 25.23 35.25 23.10 13.42 0.52 27.36 0.91
3 0.99 35.34 35.88 18.82 9.96 0.47 21.61 1.60
4 1.13 45.61 35.37 12.27 6.75 0.49 14.27 3.18
Fig. 4—Experimental apparatus for viscosity measurement.
Fig. 5—Viscosity of molten CaO-SiO
2
-Fe
x
O slags.
METALLURGICAL AND MATERIALS TRANSACTIONS B
convolution of bands related to symmetric Si-O stretch-
ing vibrations of units with variable numbers of bridging
oxygen (Q
n
: n being the number of BO).
[29–32]
Investi-
gations showed that the MF region was the overlapping
zone of the Fe-O and Si-O vibration. It was interpreted
as the convolution of bands related to symmetric Fe-O
stretching vibrations of FeO
4
and FeO
6
units
[33–36]
and
the convolution of bands related to Si-O stretching
vibrations and breathing modes of three- and four-mem-
bered ring structures of [SiO
4
] tetrahedrons.
[29–32]
Fig-
ure 6 shows that the peak intensity at 500 to 780 cm
1
steadily decreases with increasing Ca/Fe ratio, but it
does not show the obvious band related to the Si-O
vibration. Consequently, the MF region is deduced to be
mainly related to the Fe-O vibration. For the LF region,
it is usually attributed to the bending vibration involving
T-O-T bridging oxygen relative to almost stationary
four-fold coordinat ed cations in the TO
4
units, where T
refers to the fourfold cation (Si
4+
,Fe
3+
).
[37–39]
Based on the above results, Fe
3+
coordinated with four
oxygen atoms, as a network former, forms a [FeO
4
]
tetrahedron, and Fe
3+
coordinated with six oxygen
atoms, as a network modifier, form s a [FeO
6
] octahedr on.
However, the vibration related to Fe
2+
is not detected in
the Raman spectra, so Fe
2+
is considered a non-frame-
work cation, which acts as a charge compensation in the
molten slag. Similar results were obtained from Mysen’s
research.
[10]
In the present slags, the peak intensity of the
MF region decreases with varying Ca/Fe ratios, indicat-
ing that the structural beh avior of Fe
3+
has changed.
Meanwhile, the shifting shoulders of the envelope peak in
the HF region show that the different types of SiO
4
units
have also changed. These variations in Raman spectra can
affect the degree of polyme rization of the melt and thereby
alter the viscosity of molten slag. The structural evolu-
tions of Si
4+
and Fe
3+
will be further discussed by the
deconvolution method of Raman spectra.
Fig. 6—Raman spectra of quenched slags.
Fig. 7—Typical deconvolution of Raman spectra.
Fig. 8—Deconvolution result of Raman spectra.
Fig. 9—Fraction of structural units.
METALLURGICAL AND MATERIALS TRANSACTIONS B
In general, the measured curves of Raman spectra are
not on the same horizont al line because of the fluores-
cence effect. Consequently, baselines of curves over the
Raman shift of 500 to 1200 cm
1
were first subtracted.
Thereafter, the spectra were fitted using the Gauss ian
deconvolution function in the Origin 9.0 software. In
our fits, the band position, width and intensity were
treated as independent variables. The spectra were
statistically treated by minimization of residuals. What
is more, the characteristic peaks obtained from the
deconvolution results could be used to calculate the
corresponding areas by the integral method. The ratio of
the integral area to the sum of all the characteristic
peaks could give the fraction of a specific structural unit
in the molten slag. The typical dec onvolution of the
Raman spectra is shown in Figure 7. Currently, this
method has been widely used by scholars in the
deconvolution of Raman spectra.
[40–43]
In addition, some studies revealed that the Fe-O
vibration could probably exist in the HF region. For
example, Genova et al.
[44,45]
named the band at
~ 970 cm
1
of Raman spectra the ‘‘Fe
3+
band,’’ and
Muro et al.
[46]
reported that the band at ~ 980 cm
1
was
attributed to the anti-symmetric coupled mode of
FeO
4
-SiO
4
unit. In Figure 7, there is a weak shoulder
near 980 cm
1
in samples 1, 2 and 3. If an extra line
parameter denoting the Si-O-Fe bond is added at
980 cm
1
during the curve-fitting process, this peak
obtained from the deconvolution result will be covered
by the Q
2
band and its content is relatively less.
According to the result, the Si-O-Fe bond is considered
to be possibly derived from the Fe-containing Q
2
unit
because of the interconnecti on of FeO
4
and Q
2
units.
Consequently, the Raman spectra were deconvoluted
again without separately considering the assignment of
the Fe-O-Si bond.
The deconvolution results are shown in Figure 8. The
peaks near 610, 670, 840, 915, 965 and 1020 cm
1
were
confirmed as the structural units of FeO
6
, FeO
4
,Q
0
(SiO
4
), Q
1
(Si
2
O
7
), Q
2
(Si
2
O
6
) and Q
3
(Si
2
O
5
), respec-
tively.
[29–36]
The relative area fractions obtained from
the deconvolution results are shown in Figure 9.
The slag composition is a process of fixed SiO
2
content
and decreasing total iron content. Because the SiO
2
/
Fe
2
O
3
ratio increases, the relative fraction of the SiO
4
unit
obviously increases and the relative fractions of Fe-O
structural units ([FeO
4
] tetrahedron and [FeO
6
] octahe-
dron) steadily decrease. However, the relative fractions of
[SiO
4
] tetrahedrons with variable numbers of bridging
oxygen (Q
0
,Q
1
,Q
2
and Q
3
) and different types of Fe-O
structural units also have a significant change.
With the Ca/Fe ratio increasing from 0.40 to 1.60, the
most polymerized units of [SiO
4
] tetrahedrons (Q
3
and
Q
2
) de crease and the small polymerized units of [SiO
4
]
tetrahedrons (Q
1
and Q
0
) increase. Consequently, the
number of non-bridging oxygens increases and the
degree of depolymerization of molten slag decreases,
resulting in decreasing viscosity. Obviously, the reason
for depolymerization is that the O
2
ions dissociated
from CaO can cut off the Si-O-Si bonds of [SiO
4
]
tetrahedrons, causing SiO
4
units to depolymerize from
Q
3
to Q
0
. The depolymerization process is represented
by Eq. [1], which coincides with the depolymerization
mechanism proposed by Mysen et al.
[10]
Under the condition of increasing Ca/Fe ratio from 0.40
to 1.60, the sum of relative fractions of the [FeO
4
]
tetrahedron and [FeO
6
] octahedron gradually decreases,
but the FeO
4
/FeO
6
ratio increases markedly. In this stage,
most O
2
ions enter [SiO
4
] tetrahedrons, and only a small
part of O
2
ions surround Fe
3+
. With the increasing O
2
dissociated from CaO, Fe
3+
tends to combine with O
2
,
forming a [FeO
4
] tetrahedron, which promotes an increase
Fig. 10—Schematic diagram of the structural evolution of molten slag.
Si
2
O
5
(Q
3
, sheet)
Si
4
O
11
(Q
2
, double chain)
2Si
2
O
6
(Q
2
, single chain)
4SiO
4
(Q
0
, monomer)
2Si
2
O
6
(Q
2
, ring)
2Si
2
O
7
(Q
1
, dimer)
O
2-
2O
2-
O
2-
2O
2-
2O
2-
½1
METALLURGICAL AND MATERIALS TRANSACTIONS B
in the FeO
4
/FeO
6
ratio. Although the net-like structures
formed by Fe
3+
increase, depolymerization of the [SiO
4
]
tetrahedron is the main reason for decreasing viscosity.
Similar results were found in Ru
¨
ssel’s research.
[47]
With further increasing of the Ca/Fe ratio from 1.60 to
3.18, the [SiO
4
] tetrahedron shows the same depolymer-
ization behavior as the lower Ca/Fe ratio of molten slag.
However, the increasing fractions of Q
0
and Q
1
are less in
this stage, indicating that the effect of O
2
on the
depolymerization of the [SiO
4
] tetrahedron becomes
weaker when the Ca/Fe ratio is 3.18. This phenomenon
coincides with a slowly decreasing trend of viscosity of
molten slag over the range of Ca/Fe = 1.60 to 3.18.
Meanwhile, it is also observed that the relative fraction
of the [FeO
4
] tetrahedron decreases significantly and the
relative fraction of the [FeO
6
] octahedron stays constant.
The possible reason for the decreasing FeO
4
/FeO
6
ratio can
be explained as follows. When the Ca/Fe ratio of molten
slag is 1.60, much more non-bridging oxygen exists in the
melt structure, indicating that the degree of polymerization
of molten slag is in a lower state. With further increasing of
the Ca/Fe ratio, the O
2
in molten slag is relatively
excessive, which can depolymerize SiO
4
and FeO
4
groups
to simpler units such as SiO
4
and FeO
4
monomers. Because
the bond strength of Si-O (799.6 KJ/mol) is stronger than
that of Fe-O (390 KJ/mol), the SiO
4
monomer formed by
the Si-O bond is relatively compact while the FeO
4
monomer formed by the Fe-O bond is relatively loose.
Excessive O
2
can be more easily incorporated into the
FeO
4
monomer to form a FeO
6
octahedron, which leads to
better stabilization of the [FeO
6
] octahedron. Therefore,
increasing of the Ca/Fe ratio over the range of 1.60 to 3.18
favors the depolymerization of molten slag and further
decreases the viscosity of molten slag. A similar result has
been reported by Vada
´
sz et al.
[48]
who considered that the
coordination of central ferric atoms might be changed from
a tetrahedron to octahedron depending on the surplus of
free oxygen ions in the complex Fe
3+
anions. Bowker
et al.
[49]
found that Fe
3+
behaved primarily as a network
modifier in very low iron glasses, which also supports the
present experimental result.
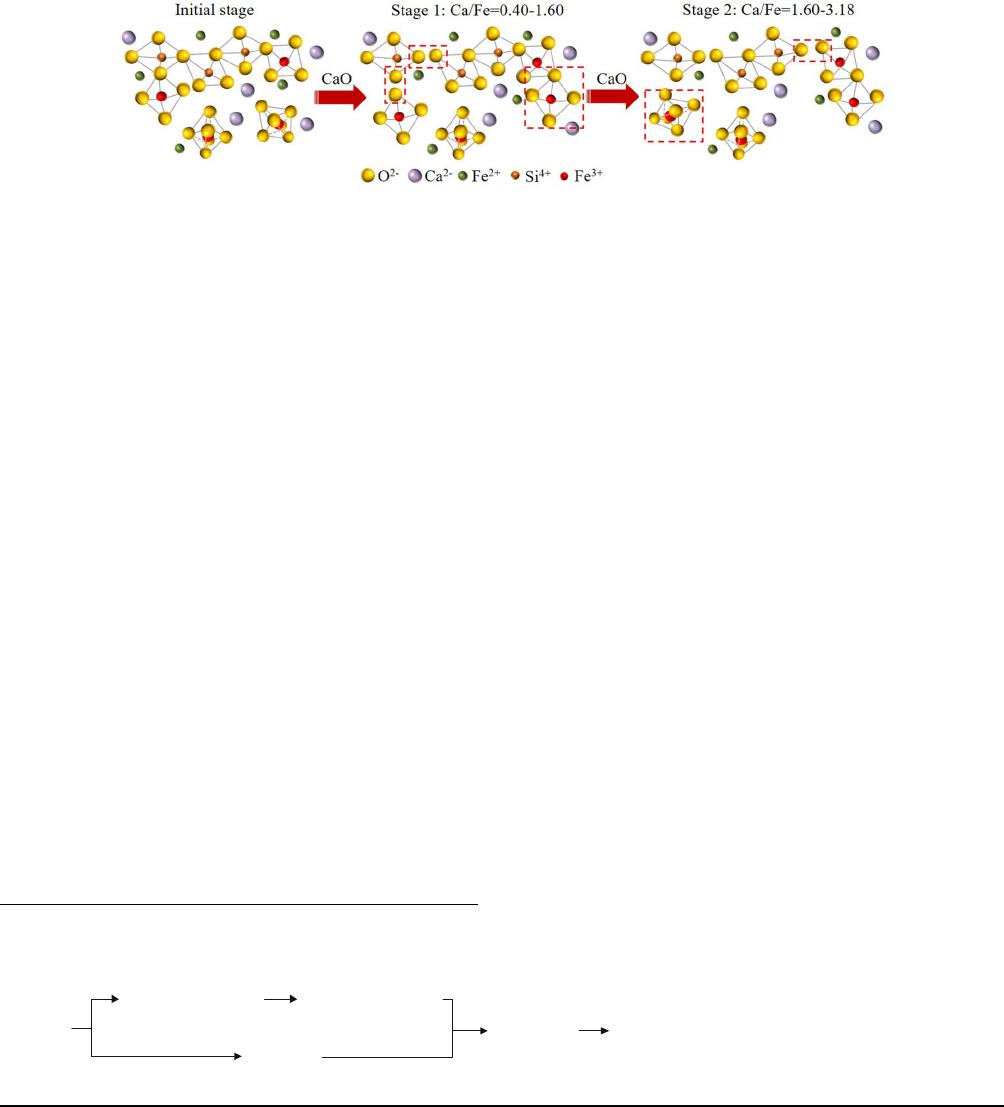
The schematic diagram of the structural behaviors of
Si
4+
and Fe
3+
in the melt are descri bed in Figure 10.
The structural evolution of molten slag is divided into
two stages in terms of different structural behaviors of
Fe
3+
. The Ca/Fe ratio increasing from 0.40 to 1.60 is
considered the first stage. In this stage, the [SiO
4
]
tetrahedron gradually depolymerizes from Q
3
to Q
0
and
the FeO
4
/FeO
6
ratio increases progressively. The Ca/Fe
ratio increasing from 1.60 to 3.18 is considered the
second stage. The [SiO
4
] tetrahedron further depoly-
merizes while the FeO
4
/FeO
6
ratio decreases. In these
two stages, the degree of polymerization of molten slag
gradually decreases, resulting in a continuous decrease
in the viscos ity of the molten slag.
C. Relationship Between Viscosity and Structure
The above results show a strong relationship between
the viscosity of molten slag and its structure. The higher
the degree of polymerization of the melt is, the larger the
viscosity of the molten slag is. Generally, three forms of
oxygen (brid ge oxygen O
0
, terminal oxygen O
and free
oxygen O
2
) are used to represent the degree of poly-
merization of molten slag. By adding some basic oxides
(such as CaO, Na
2
O) to silicate slag, O
and O
2
can be
generated by cutting off the bridging oxygen bonds of the
[SiO
4
] tetrahedron. O
and O
2
have greater fluidity than
O
0
because the oxygen is not connected to Si. Hence, the
numbers of O
and O
2
determine the viscosity of molten
slag. Nakamoto et al.
[50,51]
proposed a model for esti-
mating the viscosity of the silicate melt according to the
oxygen bonds. However, the viscosity model for the
Fe
2
O
3
-bearing silicate melt has not been studied so far. In
the present work, to establish a quantitative relationship
between the viscosity of molten slag and structure, the
viscosity estimation model for the CaO-SiO
2
-Fe
x
O slag
system is developed by applying the deconvolution results
of the Raman spectra.
For the silicate slag containing Fe
2
O
3
,Fe
3+
acts as
network former and can form Fe-O-Fe bonds (Fe-O
0
).
Therefore, when the chemical bonds of oxygen are
estimated, the Fe-O
0
bond should also be considered in
the viscosity equation proposed by Nakamoto et al.
except for three kinds of oxygen bonds related to Si. The
equation to calculate the viscosity of CaO-SiO
2
-Fe
x
O
slag is modified as follows:
g ¼ A expðE
v
=RTÞ½2
E
v
¼ E= 1 þ
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
X
i
a
i
NðO
þ O
2
Þ
i
þ
X
j
a
j
NðFe-O
0
Þ
j
q
½3
where E
v
is the activation energy; a
i
and a
j
are the
bond parameters of the slag component except SiO
2
and the non-frame cations, respectively; bond parame-
ters of CaO, FeO, Fe
2
O
3
,Ca
2+
and Fe
2+
are 4.00,
6.05, 1.00, 1.46 and 3.15,
[52]
respectively; N(O
+O
2
)
is the fraction of O
and O
2
, and N(Fe-O
0
) is the
fraction of O
0
in the [FeO
4
] tetrahedron; A is constant,
4.8 9 10
6
; E is the activation energy of pure SiO
2
,
5.21 9 10
5
J/mol.
The values of N(O
+O
2
) and N(Fe-O
0
) can be
calculated by relative fractions of structural units
obtained from Raman spectra instead of the evaluation
of the thermodynamic cell model proposed by Gaye
et al.
[53,54]
The calculated equations of N(O
+O
2
)
and N(Fe-O
0
) are shown in Eqs. [4] through [6] and the
calculated values are as shown in Table II.
NðO
þ O
2
Þ
i
¼ðN
O
NðSi - O
0
ÞNðFe - O
0
ÞÞ
n
i
=n
ðCaOþFeOþFe
2
O
3
Þ
½4
NðFe - O
0
Þ
j
¼ NðFeO
4
Þn
j
=n
ðCa
2þ
þFe
2þ
Þ
½5
NðSi-O
0
Þ¼3NðQ
3
Þþ2NðQ
2
ÞþNðQ
1
Þ; ½6
where i denotes CaO, FeO or Fe
2
O
3
; j denotes Ca
2+
and Fe
2+
; n
i
, n
j
denotes the fraction of i , j, respectively.
METALLURGICAL AND MATERIALS TRANSACTIONS B

The calculated viscosity obtained through Eqs. [2]and
[3] is shown in Figure 5. It is observed that the
calculated viscosity is in agreement with the measured
viscosity. The mean deviation (D) between the calculated
and experimental viscosities as defined in Eq. [7]is
shown in Table II.
D ¼ð1=NÞ
X
g
cal
g
exp
=g
exp
100 ð%Þ½7
where g
cal
and g
exp
are the calculated and measured
viscosities, respectively; N is the test number of
viscosity.
The mean deviations of all slags are almost within
20 pct, which is accep table for estimation of viscosity,
considering the reported experimental uncertainties of
25 pct. These results confirm that Eqs. [2] and [3]can
represent a reasonable estimation of viscosity in
CaO-SiO
2
-Fe
x
O slag systems during the early period
of basic oxygen steelmaking. The model is very useful
for estimating the viscosity of converter slag during the
early smelting period. The converter slag designer can
use this model to produce an appropriate slag that can
optimize the smelting performance to attain a satisfac-
tory metallurgical effect. Meanwhile, the estimation
model for viscosity can provide theoretical guidance
for designing the slagging route for basic oxygen
steelmaking.
IV. CONCLUSIONS
The viscosity of CaO-SiO
2
-Fe
x
O slags during the
initial period of basic oxygen steelmaking was analyzed
in terms of the structure of molten slags. The typical
conclusions are summarized as follows:
1. The viscosity of CaO-SiO
2
-Fe
x
O slags decreased
continuously with increasing Ca/Fe ratio, and the
Ca/Fe ratio showed an obvious influence on the vis-
cosity of molten slags. A more pronounced effect of
increasing Ca/Fe ratio on the decrease of viscosity
was revealed in the range of Ca/Fe = 0.40 to 1.61.
2. When the Ca/Fe ratio increased from 0.40 to 1.61,
increasing O
2
led to the depolymerization of [SiO
4
]
tetrahedrons from Q
3
to Q
0
units and an increasing
FeO
4
/FeO
6
ratio. Because depolymerization of [SiO
4
]
tetrahedrons was the main reaction, the degree of
polymerization of the molten slag gradually de-
creased and thereby decreased the viscosity of molten
slag in this stage.
3. With further increasing of the Ca/Fe ratio from 1.61
to 3.18, [SiO
4
] tetrahedrons were further depolymer-
ized, and more O
2
ions reacted with [FeO
4
] tetra-
hedrons to form [FeO
6
] octahedrons, resulting in a
decreasing FeO
4
/FeO
6
ratio. The variations of both
Si-O and Fe-O structural units caused a decrease in
the viscosity of molten slag.
4. The viscosity estimation model was established by
considering the Fe-O
0
in the melt structure and suc-
cessfully applied to the CaO-SiO
2
-Fe
x
O-based slag
system. In the model, the concentrations of bridging
oxygen, terminal oxygen and free oxygen were cal-
culated by decon volution results instead of estima-
tion derived from the thermodynamic cell model. The
model can estimate the viscosity of converter slag
during the early period of basic oxygen steelmaking
and design a reasonable converter slag to optimize
smelting perfor mance.
ACKNOWLEDGMENTS
This work was supp orted by the National Natural
Science Foundation of China (Grant Nos. 51674069,
51974075), the National Key R & D Program of Chi-
na (Grant No. 2017YFC0805100), the Open Funds of
State Key Laboratory of Metal Material for Marine
Equipment and Application (SKLMEA-K201911) and
the Fundamental Research Funds for the Central
Universities of China (Grant Nos. N182506001,
N180725008).
REFERENCES
1. J.J. Parka and R.J. Fruehan: Metall. Mater. Trans. B, 1991,
vol. 22B, pp. 39–46.
2. K. Gu, N. Dogan, and K.S. Coley: Metall. Mater. Trans. B, 2017,
vol. 48B, pp. 2595–2606.
3. J. Diao, P. Gu, D.M. Liu, L. Jiang, C. Wang, and B. Xie: JOM,
2017, vol. 69, pp. 1745–50.
4. G.H. Zhuang, K.C. Chou, Q.G. Xue, and K.C. Mills: Metall.
Mater. Trans. B, 2012, vol. 43B, pp. 64–72.
5. G.H. Kim and I. Sohn: J. Non-Cryst. Solids, 2012, vol. 358,
pp. 1530–37.
6. G.H. Kim, H. Matsuura, F. Tsukihashi, W.L. Wang, D.J. Min,
and I. Sohn: Metall. Mater. Trans. B, 2013, vol. 44B, pp. 5–12.
7. P.C. Li and X.J. Ning: Metall. Mater. Trans. B, 2016, vol. 47B,
pp. 446–57.
8. C. Feng, J. Tang, L.H. Gao, Z.G. Liu, and M.S. Chu: ISIJ Int.,
2019, vol. 59, pp. 31–38.
9. Y.M. Gao, S.B. Wang, C. Hong, X.J. Ma, and F. Yang: Int. J.
Miner. Metall. Mater., 2014, vol. 21, pp. 353–62.
10. B.O. Mysen, D. Virgo, and C.M. Scarfe: Am. Mineral., 1980,
vol. 65, pp. 690–710.
Table II. Fractions of Different Oxygen Atom Types and Mean Deviation of Viscosity
Sample NðO
+O
2
Þ
CaO
NðO
+O
2
Þ
FeO
NðO
+O
2
Þ
Fe
2
O
3
NðFe - O
0
Þ
Ca
2þ NðFe - O
0
Þ
Fe
2þ
D (Pct)
1 0.148 0.217 0.057 0.069 0.113 5.556
2 0.246 0.168 0.045 0.106 0.075 6.358
3 0.440 0.112 0.031 0.086 0.037 16.102
4 0.516 0.108 0.027 0.072 0.015 16.162
METALLURGICAL AND MATERIALS TRANSACTIONS B
11. D. Virgo and B.O. Mysen: Phys. Chem. Mineral., 1985, vol. 12,
pp. 65–76.
12. Z.J. Wang, Y.Q. Sun, S. Sridhar, M. Zhang, M. Guo, and Z.T.
Zhang: Metall. Mater. Trans. B, 2015, vol. 46B, pp. 2246–54.
13. T.F. Cooney and S.K. Sharma: J. Non-Cryst. Solids, 1990,
vol. 122, pp. 10–32.
14. G.A. Waychunas, G.E. Brown, C.W. Ponader, and W.E. Jacksom:
Nature, 1988, vol. 332, pp. 251–53.
15. W. Wu, Z.S. Zou, and Z.H. Guo: J. Iron Steel Res., 2004, vol. 16,
pp. 21–24.
16. M.Y. Zhu: Modern Metallurgical Technology, 1st ed., Beijing
Industrial Press, Beijing, 2011, pp. 207–309.
17. Y.Z. Wang, Y. Zhang, and W.H. Zhang: The Process and Equip-
ment of Oxygen Top Blown Converter Steelmaking, 2nd ed., Me-
tallurgical Industry Press, Beijing, 1983, pp. 33–40.
18. S. Caric
´
, L. Marinkov, and J. Slivka: Phys. Stat. Sol., 1975,
vol. 13, pp. 263–68.
19. J.A. Duff: J. Non-Cryst. Solids, 1996, vol. 196, pp. 45–50.
20. T. Osugi, S. Sukenaga, Y. Inatomi, Y. Gonda, N. Saito, and K.
Nakashima: ISIJ Int., 2013, vol. 53, pp. 185–90.
21. B.O. Mysen: Geochim. Cosmochim. Acta, 2006, vol. 70, pp. 2337–
53.
22. E.J. Jung and D.J. Min: Steel Res. Int., 2012, vol. 83, pp. 705–11.
23. M. Taylor, G.E.B. Jr and P.M. Fenn: Geochim. Cosmochim. Acta,
1980, vol. 44, pp. 109-17.
24. F.A. Seifert, B.O. Mysen, and D. Virgo: Geochim. Cosmochim.
Acta, 1981, vol. 45, pp. 1879–84.
25. K. Seki and F. Oeters: Trans. Iron Steel Inst. Jpn., 1984, vol. 24,
pp. 445–54.
26. M. Chen and B.J. Zhao: Metall. Mater. Trans. B, 2015, vol. 46B,
pp. 577–84.
27. F.Z. Ji, D. Sichen, and S. Seetharaman: Metall. Mater. Trans. B,
1997, vol. 28B, pp. 827–34.
28. M. Suzuki and E. Jak: Proc. VIIII Int. Conf. Molten Slags Fluxes
Salt, Beijing, 2012, pp. 68–83.
29. B.O. Mysen, D. Virgo, W.J. Harrison, and C.M. Scarfe: Am.
Mineral., 1980, vol. 65, pp. 900–14.
30. P.F. McMillan: Am. Mineral., 1984, vol. 69, pp. 622–44.
31. T. Furukawa, K.E. Fox, and W.B. White: J. Chem. Phys., 1981,
vol. 75, pp. 3226–37.
32. F.L. Galeene: Solid State Commun., 1982, vol. 44, pp. 1037–40.
33. B.O. Mysen, F.J. Ryerson, and D. Virgo: Am. Mineral., 1980,
vol. 65, pp. 1150–65.
34. G. Lucazeau, N. Sergent, T. Pagnier, A. Shaula, V. Kharton, and
F.M.B. Marques: J. Raman Spectrosc., 2007, vol. 38, pp. 21–33.
35. R. Iordanova, Y. Dimitriev, V. Dimitrov, and D. Klissurski: J.
Non-Cryst. Solids, 1994, vol. 167, pp. 74–80.
36. Z.J. Wang, Q.F. Shu, S. Sridhar, M. Zhang, M. Guo, and Z.T.
Zhang: Metall. Mater. Trans. B
, 2015, vol. 46B, pp. 758–65.
37. R.M. Santos, D. Ling, A. Sarvaramini, M. Guo, J. Elsen, F.
Larachi, G. Beaudoin, B. Blapain, and T.V. Gerven: Chem. Eng.
J., 2012, vol. 203, pp. 239–50.
38. A.A. Francis: J. Am. Ceram. Soc., 2005, vol. 88, pp. 1859–63.
39. A.A. Francis: Mater. Res. Bull., 2006, vol. 41, pp. 1146–54.
40. J.H. Park: J. Non-Cryst. Solids, 2012, vol. 358, pp. 3096–3102.
41. S. Sukenaga, N. Saito, K. Kawakami, and K. Nakashima: ISIJ
Int., 2006, vol. 46, pp. 352–58.
42. J. Yang, J.Q. Zhang, Y. Sasaki, O. Ostrovski, C. Zhang, D. Cai,
and Y. Kashiwaya: Metall. Mater. Trans. B, 2017, vol. 48B,
pp. 2077–91.
43. J. Qi, C.J. Liu, and M.F. Jiang: J. Non-Cryst. Solids, 2017,
vol. 475, pp. 101–07.
44. D.D. Genova, S. Sicola, C. Ramano, A. Vona, and S. Fanara:
Chem. Geol., 2017, vol. 457, pp. 76–86.
45. D.D. Genova, D. Morgavi, K. Hess, D.R. Neuville, N. Borovkov,
D. Perugini, and D.B. Digwell: J. Raman Spectrosc., 2015, vol. 46,
pp. 1235–1244.
46. A.D. Muro, N. Mtrich, M. Mercier, D. Giordano, D. Massare,
and G. Montagnac: Chem. Geol., 2009, vol. 259, pp. 78–88.
47. C. Ru
¨
ssel and A. Wiedenroth: Chem. Geol., 2004, vol. 213,
pp. 125–35.
48. P. Vada
´
sz, M. Havlı
´
k, and V. Daneˆ k: Can. Metall. Quart., 2000,
vol. 39, pp. 143–52.
49. J.C. Bowker, C.H. Lupis, and P.A. Flinn: Can. Metall. Quart.,
1981, vol. 20, pp. 69–78.
50. M. Nakamoto, J. Lee, and T. Tanaka: ISIJ Int., 2005, vol. 45,
pp. 651–56.
51. M. Nakamoto, T. Tanaka, J. Lee, and T. Usui: ISIJ Int., 2004,
vol. 44, pp. 2115–19.
52. M. Nakamoto, Y. Miyabayashi, L. Holappa, and T. Tanaka: ISIJ
Int., 2007, vol. 47, pp. 1409–15.
53. H. Gaye and J. Welfringer: Proc. 2nd Int. Symp. Metall. Slags and
Fluxes, TMA-AIME, Warrendale, PA, 1984, pp. 357–75.
54. H. Gaye, J. Lehmann, T. Matsumiya and W. Yamada: 4th Int.
Conf. on Molten Slags and Fluxes, ISIJ, Tokyo, 1992, pp. 103–08.
Publisher’s Note Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations.
METALLURGICAL AND MATERIALS TRANSACTIONS B
