
FISH PARASITOLOGY - SHORT COMMUNICATION
First report of Myxobolus episquamalis Egusa, Maeno & Sorimachi,
1990 (Myxozoa: Bivalvulida) in Lebranche mullet Mugil liza
Valenciennes, 1836 (Teleostei: Mugiliformes) from Neotropical region
Rayane Duarte
1
& Bruna Reich Martinatti
2
& Águida Aparecida de Oliveira
3
& Jhon Lennon Genovez-Oliveira
4
&
Viviane Moreira de Lima
5
& Rafael de Almeida Tubino
4,5
& Bruno Pereira Berto
5
& Michelle Daniele Santos-Clapp
5
Received: 12 January 2020 /Accepted: 6 July 2020
#
Springer-Verlag GmbH Germany, part of Springer Nature 2020
Abstract
In the current study, Myxobolus episquamalis Egusa, Maeno & Sorimachi, 1990 (Myxozoa: Bivalvulida) is reported from the
Lebranche mullet Mugil liza Valenciennes, 1836 in the estuarine region of the Maricá Lagoon, State of the Rio de Janeiro,
southeastern Brazil. To date, this myxozoan species was reported in mullets from Asia, Africa, Europe, and Oceania. The
characteristics of M. episquamalis previously reported are similar to the findings of the present study. DNA sequences of the
nuclear small subunit ribosomal DNA (SSU rDNA) had 99.7–100% similarity with the sequences of M. episquamalis from North
Africa and Asia. Therefore, strong morphological and molecular similarities ensure the identification of M. episquamalis in the
current study. Finally, this finding records a new host and locality, revealing the worldwide distribution of this myxozoan species.
Keywords Morphology
.
Sequencing
.
Myxozoa
.
Myxobolus episquamalis
.
Mugil liza
.
Maricá Lagoon
Introduction
The Lebranche mullet Mugil liza Valenciennes, 1836 (syn.
M. platanus Günther, 1880) is a catadromous pelagic fish
from tropical and subtropical waters, mainly from estuarine
coastal regions. It is distributed from the USA to Argentina,
occurring in saltwater, freshwater, and brackish water (Froese
and Pauly 2019).
Myxozoans are common parasites of marine and freshwa-
ter fish, rarely of amphibians and reptiles, and exceptionally of
birds and mammals, representing an important pathogenic
group with a worldwide distribution (Lom and Dyková
2006). Among myxozoans, the genus Myxobolus Bütschli,
1882 has the largest known diversity. In a revision of Eiras
et al. (2014), a total of 905 Myxobolus spp. were checked and
listed. Approximately 90 species of myxozoans have been
identified in South America, of which 41 are Myxobolus
spp., most of them from Brazil. To date, there are three reports
of Myxobolus spp. from M. liza:(1)Myxobolus sp. from scales
of M. liza from the coast of Rio de Janeiro State, Brazil (Knoff
and Serra-Freire 1993); (2) Myxobolus platanus Eiras, Abreu,
Robaldo & Pereira Júnior, 2007 described infecting the spleen
of M. liza from lagoons in the State of Rio Grande do Sul,
Brazil (Eiras et al. 2007); and (3) M yxobolus s aladensis
Marcotegui & Martorelli, 2017 which was described parasit-
izing the gills of M. liza from Buenos Aires, Argen tina
(Marcotegui and Martorelli 2017).
In the present study, Myxobolus episquamalis Egusa, Maeno
&Sorimachi,1990, originally described from the flathead gray
mullet Mugil cephalus Linnaeus, 1758 in Japan (Egusa et al.
Section Editor: Astrid Holzer
* Bruno Pereira Berto
berto.ufrrj@gmail.com
1
Programa de Pós-Graduação em Ciências Veterinárias, Instituto de
Veterinária (IV), Universidade Federal Rural do Rio de Janeiro
(UFRRJ), BR-465 km 7, Seropédica, RJ 23897-000, Brazil
2
Curso de Graduação em Medicina Veterinária, IV, UFRRJ,
BR-465 km 7, Seropédica, RJ 23897-000, Brazil
3
Departamento de Microbiologia e Imunologia Veterinária, IV,
UFRRJ, BR-465 km 7, Seropédica, RJ 23897-000, Brazil
4
Programa de Pós-Graduação em Biologia Animal, Instituto de
Ciências Biológicas e da Saúde (ICBS), UFRRJ, BR-465 km 7,
Seropédica, RJ 23897-000, Brazil
5
Departamento de Biologia Animal, ICBS, UFRRJ, BR-465 km 7,
Seropédica, RJ 23897-000, Brazil
Parasitology Research
https://doi.org/10.1007/s00436-020-06803-3

1990), was identified from a new host M. liza, from the Maricá
Lagoon in southeastern Brazil, Neotropical region.
Materials and methods
Sample collection
A total of 19 specimens of M. liza (total length 31.9 cm [26–
45 cm]; 328.2 g [178–811 g]) were collected by local fisher-
men in the Maricá Lagoon (22°52′ and 23°00′ S, 43°00′ and
42°45′ W), State of Rio de Janeiro, Brazil. These specimens
were transported to the Laboratório de Biologia e Ecologia de
Parasitos (LABEPAR) of the Universidade Federal Rural do
Rio de Janeiro (UFRRJ) for parasitological analysis.
Morphological analysis
Cyst-like plasmodia on infected scales from freshly collected
fish were carefully removed and squashed for the observation
of myxozoan myxospores. Morphological observations, pho-
tomicrographs, and measurements of live myxospores were
made using an Olympus BX binocular microscope
(Olympus Optical, Tokyo, Japan) coupled to a digital camera
Eurekam 5.0 (BEL Photonics, Monza, Italy).
Histological analysis
For histological analysis, fragments of infected areas were
carefully removed from the scales with a scalpel. These frag-
ments were fixed in 10% neutral buffered formalin and em-
bedded in paraffin blocks. Five mic rometer (μm) sections
were obtained using a manual microtome and stained with
hematoxylin-eosin.
Molecular analysis
DNA was extracted from plasmodia in PBS, using the Qiagen
DNeasy Blood and Tissue Kit (Qiagen, São Paulo, Brazil)
according to the manufacturer’s instructions. The PCR ampli-
fication of the nuclear small subunit ribosomal DNA (SSU
rDNA) was carried out from two samples (two individuals)
as previously described by Özer et al. (2016), using the
primers Henn_Myx_120F (5′ -AATCTGCTCGATTG
TAAGGG-3′ ) and Henn_Myx_2100Rev (5′ -CCGC
TCCCAAGGTATTAT-3′). For amplification, a 25 μlPCR
reaction was prepared using 1 μL of genomic DNA (<
1 μg), 12.5 μL of GoTaq® G2 Hot Start Colorless Master
Mix (Promega) (× 1), 0.25 μL of each primer (0.2 μm), and
11 μL of nuclease free water. The thermal cycling protocol
was as follows: an initial denaturation step at 94 °C for 5 min,
followed by 35 cycles at 94 °C for 30 s, 51 °C for 45 s, and
72 °C for 2 min and 30 s; completed with terminal extension at
72 °C for 5 min; and then stored at 4 °C. The amplicon was
purified using the ReliaPrep™ DNA Clean-up and
Concentration System (Promega Corporation, São Paulo,
Brazil). The PCR product was sequenced using the internal
forward and reverse primers, NS3 (5′ -GCAAGTCT
GGTGCCAGCAGCC-3′ )andNS4(5′ -CTTC
CGTCAATTCCTTTAAG-3′)(Whiteetal.1990), by
Ludwig Biotechnology, were an ABI-Prism 3500 Genetic
Analyzer (Applied Biosystems, Foster City, California) was
used for Sanger sequencing. The results of the sequencing
reactions were analyzed and edited using the program
Chromas 2.6. The sequence of the Myxobolus species obtain-
ed from M. liza was compar ed with se quences o f other
myxozoans available in the GenBank database, using t he
Basic Local Alignment Search Tool (BLAST).
Results and discussion
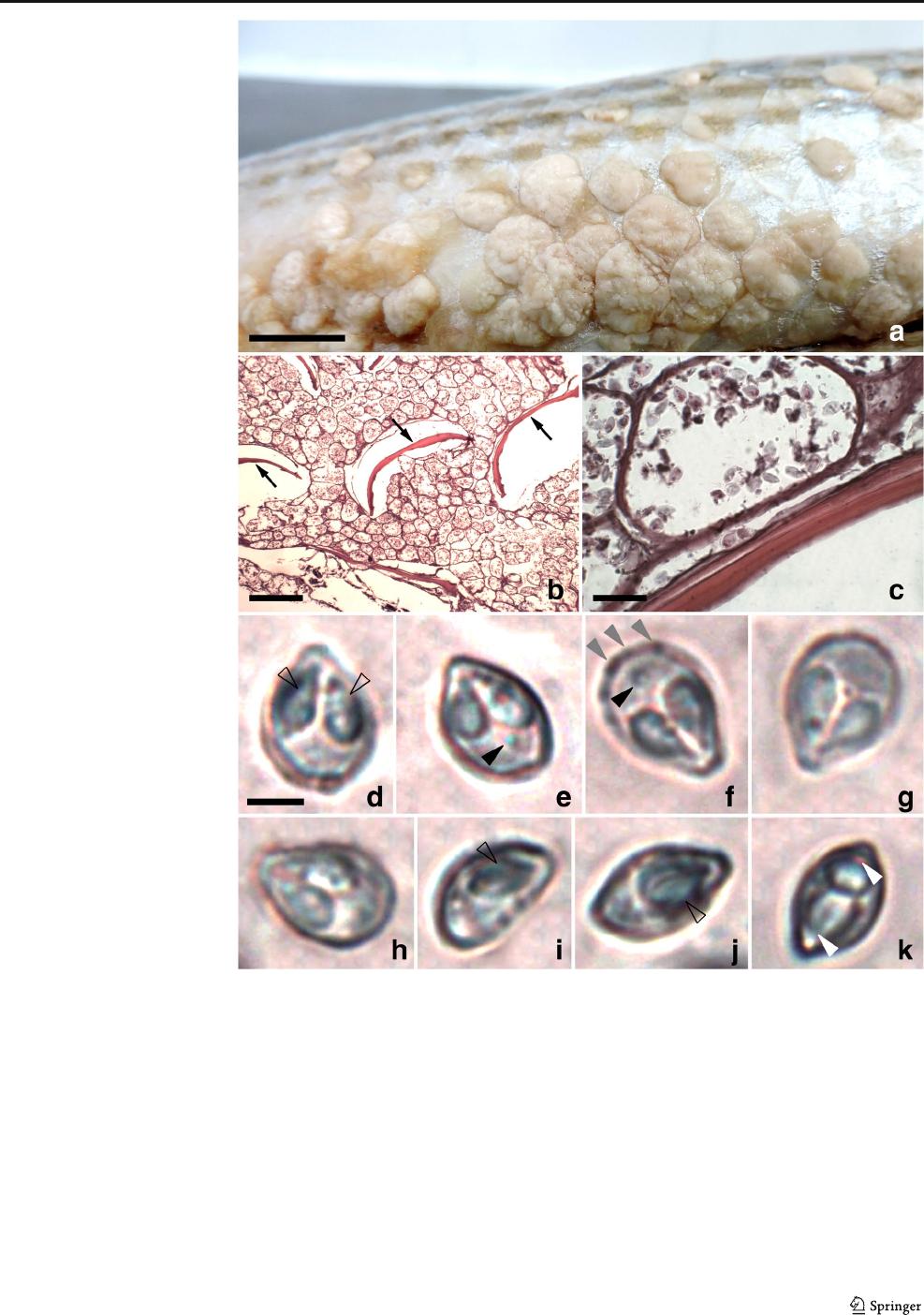
Nineteen M. liza were examined, and one was infected by a
myxozoan species that was morphologically identified as
M. episquamalis (Fig. 1). The cystic masses were observed
over the scales of the body and on the tail fin, but they were
concentrated on the ventral region of the fish, measuring 9–
10 mm in length and 5–7 mm in width (Fig. 1a). They were
oval or irregularly shaped, spongy, and whitish, located at the
dorsal surface of the scales, mainly near the insertion area in
the integument. A thin and dense collagen capsule was sur-
rounding the plasmodia. In some plasmodia, mature
myxospores were visible. Whitish irregular ellipsoidal and
polysporic plasmodia measuring 50–180 μm in length and
40–110 μm in width were observed in these cysts (Figs. 1b–
c). The mature myxospores (Fig. 1d–k) were broadly ovoid in
front view, tapering toward at the anterior end, measuring
8.2 μm(7.5–9.1) in length, 6.0 μm(5.3–6.9) in width, and
4.8 μm(4.6–5.5) in thickness. Along the sutural edge, several
triangular markings barely discernible were observe d
(Fig. 1f). Two pyriform polar capsules were present, frequent-
ly with unequal sizes, measuring 4.5 μm(3.8–5.3) in length
and 2.2 μm(1.8–2.6) in width. The polar filaments were in-
discernible within polar capsules (Fig. 1d–k). In the minority
of myxospores, a relatively large iodiophilous vacuole could
be observed in the sporoplasm (Fig. 1e–f). In lateral view, the
myxospores were lenticular, and the sutural ridge was broad,
but the sutural line was indistinct (Fig. 1k).
Photomicrographs, histological slide, and cysts in 70% eth-
anol were deposited in the Museu de Zoologia at the UFRRJ,
Brazil, under accession number MZURMTZ2020021.
Photomicrographs were d eposited and available (http://r1.
ufrrj.br/labicoc/colecao.html) in the Parasitology Collection
of the Laboratório de Biologia de Coccídios, at UFRRJ,
under the repository number 104-2020. Photovouchers of
the host specimens were deposited in the same collection.
Parasitol Res
The main distinguishing feature of M. episquamalis is the
tropism for epithelial tissue of scales and tail fin of mullets
(Rothwell et al. 1997). According to Salim and Desser (2000),
myxosporeans have a high degree of specificity, both for host
species and parasitized tissue. In this sense, although
M. episquamalis has been recorded in four host species, the
parasitized tissue remains the same. Furthermore, the
morphological characteristics of M. episquamalis reported
by Egusa et al. (1990) and later by other authors are similar
with the finding of the current study. Myxobolus episquamalis
parasitized hosts of the same family (Mugilidae), with the
same sites of infection and lesion pattern, besides having the
same morphology and morphometry of myxospores. The
differences observed in the current study were the new host
species an d the new locality, which reveal the worldwide
distribution of M. episquamalis. This myxozoan has been
recorded in 12 geographic regions in four continents, Asia,
Africa, Europe, and Oceania. In the current study, we report
this species for the first time from South America. In addition,
we suggest that the Myxobolus sp. reported as M. cephalus
Fig. 1 Cysts, plasmodia, and
myxospores of Myxobolus
episquamalis Egusa, Maeno &
Sorimachi, 1990 from Lebranche
mullet Mugil liza Valenciennes,
1836. a Cysts on the scales
located on the abdomen of the
fish. b Histological section of a
cyst, showing plasmodia and
fragments (arrows) of scales. c
Mature myxospores and other
sporogonic stages in plasmodia.
d–k Fresh myxospores in frontal
view (d–h)andlateralview(i–k)
showing polar capsules (empty
arrowhead), triangular markings
(gray arrowhead), vacuole (black
arrowhead), and the broad sutural
ridge (white arrowhead). Bar =
10 mm (a), 200 μm(b), 20 μm
(c), 3 μm(d–k). H&E stained (b,
c)
Parasitol Res

from Portugal by Menezes (1984)shouldbeM. episquamalis,
since this species is morphologically identical and has been
found on the scales of the mullet.
DNA amplification of the SSU rDNA of the samples of
M. episquamalis from M. liza produced DNA fragments of
1000 bp length. The seque nces of the two samples were 100%
identical to each other and to M. episquamalis (JF810537;
MK012069), reported from gray mullet M. cephalus in Asia
(Kim et al. 2013;Simsek2019), and 99.7% similar to the first
sequence of M. episquamalis (AY129312) deposited in
GenBank, obtained from M. cephalus from Tunisia, northern
Africa (Bahri et al. 2003). A representative sequence of the cur-
rent work was deposited in GenBank under the accession num-
ber MN822014. The high levels of genetic similarity between
our sequence and the sequences of M. episquamalis from North
Africa and Asia confirmed the identification of M. episquamalis
from the Lebranche mullet in southeastern Brazil.
Acknowledgments We thank the fisherman Mr. Lu cielio de Moura
Costa, in the municipality of Maricá, State of Rio de Janeiro, for giving
us the fish that were analyzed in the current study. We are also grateful to
the Laboratório de Histologia e Embriologia of the Departamento de
Biologia Animal of the UFRRJ for the preparation of histological slides.
Funding information This study was supported by a compensatory mea-
sure established by the Chevron Responsibility Adjustment Term, con-
ducted by the Federal Public Ministry, with the implementation of the
Fundo Brasileiro para a Biodiversidade (FUNBIO), in addition to the
support of the Coordenação de Aperfeiçoamento de Pessoal de Nível
Superior (CAPES), Conselho Nacional de Desenvolvimento Científico
e Tecnológico (CNPq), and Fundação Carlos Chagas Filho de Amparo à
Pesquisa do Estado do Rio de Janeiro (FAPERJ). RD and JLG-O have a
scholarship from CAPES (Grant/Award Number: 001). BRM had a
scholarship from CNPq (Grant/Award Number: PIB179/2018). BPB
has a fellowship from CNPq (Grant/Award Number: 302437/2016-9
and 303899/2019-0) and from FAPERJ (Grant/Award Number: E-26/
203.200/2016 and E-26/202.797/2019).
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of
interest.
Ethical approval Animal collection complied with the guidelines of the
Conselho Nacional de Controle de Experimentação Animal and were
approved by the Comissão de Ética no uso de Animais (CEUA/ICBS)
of the UFRRJ under protocol no. 005/2019.
References
Bahri S, Andree KB, Hedrick RP (2003) Morphological and phylogenetic
studies of marine Myxobolus spp. from mullet in Ichkeul Lake,
Tunisia. J Eukaryot Microbiol 50:463–470
Egusa S, Maeno Y, Sorimachi M (1990) A new species of Myxozoa,
Myxobolus episquamalis sp. nov. infecting the scales of the mullet,
Mugil cephalus L. Fish Pathol 25:87–391
Eiras JC, Abreu PC, Robaldo R, Pereira Junior J (2007) Myxobolus
platanus n. sp. (Myxosporea, Myxobolidae), a parasite of Mugil
platanus Günther, 1880 (Osteichthyes, Mugilidae) from Lagoa dos
Patos, RS, Brazil. Arq Bras Med Vet Zootec 59:895–898
Eiras JC, Zhang J, Molnár K (2014) Synopsis of the species of Myxobolus
Bütschli, 1882 (Myxozoa: Myxosporea, Myxobolidae) described
between 2005 and 2013. Syst Parasitol 88:11–36
Froese R, Pauly D (2019) Fishbase. Available from: http://www.fishbase.
org. Accessed 12 Jan 2019
Kim WS, Kim JH, Jang MS, Jung SJ, Oh MJ (2013) Infection of wild
mullet (Mugil cephalus) with Myxobolus episquamalis in Korea.
Parasitol Res 112:447–451
Knoff M, Serra-Freire NM (1993) Protozoários parasitos de Mugil
platanus Günther. Rev Bras Parasitol Vet 2:25–28
Lom J, Dyková I (2006) Myxozoan genera: definition and notes on tax-
onomy, life-cycle terminology and pathogenic species. Folia
Parasitol 53:1–36
Marcotegui P, Martorelli S (2017) Myxobolus saladensis sp. nov., a new
species of gill parasite of Mugil liza (Osteichthyes, Mugilidae) from
Samborombón Bay, Buenos Aires, Argentina. Iheringia Ser Zool
107:e2017026
Menezes J (1984) A case of massive cutaneous myxobolosis in wild
mullet. Bol Inst Pesca 12:71–72
Özer A, Gürkanlı CT, Özkan H, Acar G, Çiftçi Y, Yurakhno V (2016)
Molecular characterization and morphological aspects of Myxobolus
parvus (Myxozoa) from Liza saliens (Mugilidae) off the Turkish
Black Sea coasts. Parasitol Res 115:3513–3518
Rothwell JT, Virgona JL, Callinan RB, Nicholls PJ, Langdon JS (1997)
Occurrence of cutaneo us infections of Myxobolus episquamalis
(Myxozoa: Myxobolidae) in sea mullet, Mugil cephalus L, in
Australia. Aust Vet J 75:349–352
Salim KY, Desser SS (2000) Description and phylogenetic systematic of
Myxobolus ssp. from Cyprinids in Algoniun Park, Ontario. J
Eukaryot Microbiol 47:309–318
Simsek E (2019) First molecular data on Myxobolus episquamalis
(Myxozoa: Myxosporea) infecting the scale of Grey mullet (Mugil
cephalus) from Turkey. Turkiye Parazitol Derg 43:135–
142
White TJ, Burns T, Lee S, Taylor J (1990) Amplification and direct
sequencing of fungal ribosomal RNA genes for phylogenetics. In:
Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a
guide to methods and applications. Academic Press, San Diego, pp
315–322
Publisher’snoteSpringer Nature remains neutral with regard to jurisdic-
tional claims in published maps and institutional affiliations.
Parasitol Res
