
ORIGINAL PAPER
Antioxidant activity and hepatoprotective potential
of Hammada scoparia against ethanol-induced
liver injury in rats
Ezzeddine Bourogaa & Riadh Nciri &
Raoudha Mezghani-Jarraya &
Claire Racaud-Sultan & Mohamed Damak &
Abdelfattah El Feki
Received: 20 April 2012 / Accepted: 2 August 2012 / Published online: 15 August 2012
#
University of Navarra 2012
Abstract The present work was aimed at studying the
antioxidative activity and hepatoprotective effects of
methanolic extract (ME) of Hammada scoparia leaves
against ethanol-induced liver injury in male rats. The
animals were treated daily with 35 % ethanol solution
(4 gkg
−1
day
−1
) during 4 weeks. This treatment led to an
increase in the lipid peroxidation, a decrease in antiox-
idative enzymes (catalase, superoxide dismutase, and
glutathione peroxidase) in liver, and a considerable in-
crease in the serum levels of aspartate and alanine ami-
notransferase and alkaline p hospahata se. However,
treatment with ME protects efficiently the hepatic func-
tion of alcoholic rats by the considerable decrease in
aminotransferase contents in serum of ethanol-treated
rats. The glycogen synthase kinase-3 β was inhibited
after ME administration, which leads to an enhancement
of glutathione peroxidase activity in the liver and a
decrease in lipid peroxidation rate by 76 %. These
biochemical changes were consistent with histopatho-
logical observations, suggesting marked hepatoprotec-
tive effect of ME. These results strongly suggest that
treatment with methanolic extract normalizes various
biochemical parameters and protects the liver against
ethanol induced oxidative damage in rats.
Keywords Hammada scoparia
.
Methanolic extract
.
Oxidative stress
.
Hepatoprotective effect
Introduction
Alcohol abuse and alcoholism are serious current
health and socioeconomic problems throughout the
world. Despite great progre ss made in the field, the
development of suitable medications for the treatment
of alcoholism remains a challeng ing purpos e for alco-
hol research. Ethanol is almost exclusively metabo-
lized in t he cytosol and mitochondria by enzyme
catalyzed oxidative processes [26]. In fact, the liver
accounts for 90 % of alcohol metabolism and is the
most adversely affected organ [16]. Thr ee pathologi-
cally life-threatening liver diseases induced by alcohol
abuse are fatty liver (steatosis), hepatitis, and cirrhosis.
Moreover, oxidative stress is a central etiological
factor in various pathologies. In vertebrates for example,
a major defense mechanism against oxidative stress is
J Physiol Biochem (2013) 69:227–237
DOI 10.1007/s13105-012-0206-7
E. Bourogaa (*)
:
R. Nciri
:
A. El Feki
Laboratoire d’Ecophysiologie Animale,
Faculté des Sciences de Sfax,
PB 1171, 3000 Sfax, Tunisia
e-mail: [email protected]om
R. Mezghani-Jarraya
:
M. Damak
Laboratoire de Chimie des Substances Naturelles,
Faculté des Sciences de Sfax,
PB 1171, 3000 Sfax, Tunisia
C. Racaud-Sultan
INSERM, U563, Centre de Physiopathologie de Toulouse
Purpan, Université Toulouse III Paul Sabatier,
Toulouse, France
orchestrated by the transcription factor Nrf2, which
regulates the expression of antioxidant phase II genes
[24]. Some of the best known enzymatic antioxidants
are glutathione reductase, glutathione peroxydase
(GPx), catalase (CAT), and superoxide dismutase
(SOD), which convert active oxygen molecules into
non-toxic compounds [2, 25]. Alcohol-induced oxida-
tive stress is linked to the metabolism of ethanol involv-
ing b oth microsomal and mitochondrial systems.
Ethanol metabolism is directly involved in the produc-
tion of reactive oxygen species (ROS) and rea ctive
nitrogen species [21]. These form an environment fa-
vorable to oxidative stress. Ethanol treatment results in
the depletion of the endogenous antioxidants (SOD,
CAT, and GPx) in liver.
It has also been reported that oxidative stress resis-
tance is modulated by a glycogen synthase kinase-3
(GSK-3) whose two highly homologous forms in
mammals were identified as GSK-3β and GSK-3α.
GSK-3β activity, on the one hand, is regulated by
phosphorylation of the Ser
9
position (inactive form)
and Tyr
216
position (active form) [19]. On the other
hand, GSK-3β, a constitutively active serine/threonine
kinase, which was initially described as a key enzyme
involved in glycogen metabolism, is known to regu-
late diverse cell function pathways [7].
Medicinal plants or the isolated bioactive constitu-
ents form one of the major sources of raw materials for
drugs [ 3 ] in preventive and/or curative applications.
Public, academic, and government interests in tradi-
tional medicines are growing exponentially due to the
increased incidence of the adverse drug reactions and
economic burden of the modern system of medicine.
Many natural products have been reported as having
an inhibitory effect on previous ethanol absorption,
thus being an alternative to synthetic medicines in
the prevention of alcohol-provoked liver damage and
dysfunction. The leaves of Hammada scoparia (com-
monly known in Tunisia as Rimth; Family: Chenopo-
diaceae) have widely been used in traditional medicine,
prevention of several disorders as cancer, hepatitis,
inflammation, a nd obesity [9].
Our recent studies also showed that H. scoparia
leaves extract possess a molluscicidal activity of the
principal alkaloids against Galba truncatula [15] and a
potent anti-tumoral activity [4, 5].
Regarding t he ability of natural’s compoun ds to
reduce alcohol liver diseases, we proposed that hexane
extract (HE), dichloromethane extract (DE), and
methanolic extract (ME) of H. scoparia might possess
significant capacities to reduce tissue injuries induce by
alcohol in an in vivo conditio ns. For that case, we
planned to evaluate the aminotransferases and endoge-
nous antioxidants in serum and liver of rats.
Materials and methods
Plant materials
Hammada scoparia (Pomel) Iljin belongs to the Cheno-
podiaceae family and is locally known as “Rimth” in
T unisia. The plant was collected in southern Tunisia in
June 2007. A voucher specimen no. LCSN100 was de-
posited at “the Chemistry Laboratory of Natural Substan-
ces, Sfax Faculty of Science (Tunisia).” The leaves were
carefully detached from the fresh plant and air dried.
Preparation of extracts
Air-dried leaves (200 g) of H. scoparia were extracted
consecutively during 24 h each time, using a Soxhlet
apparatus by increasing various polarity solvents
(Fig. 1), hexane, dichloromethane, and methanol. All
solvents were evaporated under reduced pressure, and
the extract was dried to yield the hexane extract (HE,
1.05 % w /w), the dichloromethane extract (DE, 2.27 %
Fig. 1 Successive extractions of leaves of Hammada scoparia
using three solvents: hexane, dichloromethane, and methanol
(an increasing order of polarity)
228 E. Bourogaa et al.

w/w), and the methanolic extract (ME, 15.07 % w/w).
These extracts were stored under refrigeration for fu-
ture use. The concentration used in the experiment was
based on the dry weight of the extract.
Phytochemical screening
The preliminary phytochemical screening was per-
formed according to the Harborne method [12]. Plant
extracts (hexane, dichloromethane, and methanol)
were subjected to chemical tests for the presence of
sterols, triterpenoids, carotenoids, tropolons, quinons,
alkaloids, and flavonoids.
Determination of total phenolic content
Determination of total polyphenolic content i n the
extracts of H. scoparia leaves expressed in terms of
gallic acid was measured by the modified Folin–Cio-
calteu procedure [28]. Briefly, 1 ml of sample (1 mg/
ml) was mixed with 1 ml of Fo lin – Ciocalteu reagent.
After 3 min, 1 ml of saturated Na
2
CO
3
solution was
added to the mixture and adjusted to 10 ml with
distilled water. The reaction was kept in the dark for
90 min, after w hich the absorbance was read at
725 nm, and the total polyphenol concentration was
calculated from a calibration curve using gallic acid as
standard.
DPPH assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scav-
enging capacity of plant extracts represents the free
radical reducing activity of compounds based on one-
electron reduction. Briefly, 1.5 ml of DPPH solution
(10
−5
M, in ethanol 95 %) was incubated with 1.5 ml
of plant extract at varying concentrations (0.01−1 mg).
The reaction mixture was shaken well and incubated
in the dar k for 30 min at room temperature. The
control was prepared as above without any extract.
The absorbance of the solution was measured at
517 nm against a blank. Percentage of DPPH scav-
enged by all extracts at varying concentrations was
calculated as follows:
% Inhibition ¼ A
blank
A
sample
=A
blank
100
where A
blank
is the absorbance of the control reaction
containing all reagents except the sample, and A
sample
is the absorbance of the sample. Extract concentration
providing 50 % inhibition (IC50) of DPPH was calcu-
lated from the graph of inhibition percentage against
extract concentration. Ascorbic acid and α-tocopherol
were used as standards.
Antioxidant assay using the β-carotene bleaching
method
The oxidative losses of β-carotene/linoleic acid emul-
sion were used to assess the antioxidation ability of the
extracts of H. scoparia leaves. One milligram of β-
carotene was dissolved in 10 ml of chloroform, and
1mlβ-carotene solution was mixed with 20 mg of
linoleic acid and 200 mg of Tween 40 emulsifier in a
round-bottom flask. The chloroform was removed by
nitrogen gas, and 50 ml of oxygenated distilled water
was added slowly to the semisolid residue with a
vigorous shaking, to form an emulsion. An absorbance
at 470 nm was immediately recorded after adding 2 ml
of the sample to the emulsion, which was regarded as
t0 0 min. The round-bottom flasks were capped and
placed in an incubator at 50 °C. The absorbance at
470 nm was determined every 15 min until 120 min. A
second emulsion, consisting of 20 mg of linoleic acid
and 200 mg of Tween 40 and 50 ml of oxygenated
water, was also prepared and served as blank to zero
the spectrophotometer. The antioxidan t activity coef-
ficient (AAC) was calculated accordi ng to the follow-
ing equation:
AAC ¼ A
A 120ðÞ
A
C 120ðÞ
= A
Cð0Þ
A
C 120ðÞ
1000
where A
A(120)
is the absorbance of the antioxidant at
120 min, A
C(120)
is the absorbance of the control at
120 min, and A
C(0)
is the absorbance of the control at
0 min. The AAC was calculated from the graph of
absorbance against time. Butylated hydroxytoluene
(BHT) and α-tocopherol were used as standards.
Animals and treatments
Male Wistar rats weighing 150±25 g, obtained from
the breeding center, Central Pharm acy of Tunis
(Tunisia), were used for the study. They were housed
in cages (six animals per cage) placed in a breeding
farm maintained at 23 °C, equipped with a ventilation
system and light controlled conditions (10 h dark and
14 h light). These animals were allowed free access to
Hepatoprotective potential of H. scoparia 229

a standard dry pellet diet supplied by the Industrial
Society of Concentrates, (SICO, Sfax, Tunisia). The
rats were acclimatized to laboratory conditions for
7 days before the beginning of the experiment. The
animals were maintained in accordance with guide-
lines for animals care per the Sfax Faculty of Science,
Tunisia.
The rats were randomly divided into five groups of
six males, as follow s:
Group 1 (control) Served as untreated control and
received i.p. injections of 9‰
NaCl
Group 2 (EtOH) Orally administered with 4 g eth-
anol (35 %)/kg b.w. to induce
liver damages
Group 3 (HE) Given the same dose of ethanol
andinjectedi.p.withHEat
200 mg/kg b.w.
Group 4 (DE) Treated with ethanol and injected
by DE 200 mg/kg b.w.
Group 5 (ME) Treated with ethanol and injected
by ME 200 mg/kg b.w.
All extracts (HE, DE, and ME) were evaporated
and dissolved in ethano l/H
2
O (1/9, v/v) before admin-
istration to animals. The administration of ethanol
started 3 days bef or e treatment with plant extracts
and was maintained until the end of treatment.
At the end of the 4-week experimental period, the
rats were killed by decapitation. Blood samples were
collected, and livers were removed and weighed after
the removal of the surrounding connective tissues.
Blood analys is
The hematological parameters [red blood cell (RBC),
white blood cell (WBC), and hematocrit] were deter-
mined using an autoanalyzer (Coulter Maxem). Serum
aspartate and alanine aminotransf erases (AST and
ALT) and alkaline phosphatase (ALP), activities were
determined using commercial kits supplied by Sigma
Munich (Munich, Ge rmany) and Boehringer Man-
nheim (Mannheim, Germany).
Oxidative stress parameters
Oxidative stress analysis was performed by the
examination of thiobarbituric acid-reactive substances
(TBARS) and SOD, CAT, and GPx activities. The
preparation of the liver tissue was as follows. One gram
of liver tissue was homogenized in 2 ml TBS (50 mM
Tris, 150 mM NaCl, pH0 7.4) in an ultrasound homog-
enizer and centrifuged at 9,000 rpm for 15 min. The
supernatants were removed and stored at −80 °C for
subsequent analysis.
TBARS assay
As a marker of lipid peroxidation, the TBARS con-
centration was measu red based on the method of
Esterbauer et al. [10]. Briefly, 250 μl of the superna-
tant was mixed with 100 μl TBS buffer, 250 μlof
trichloroacetic acid–BHT (20 %− 1 %), vortexed and
then centrifuged at 1,000 rpm for 10 min. Besides,
400 μl of the collected supernatant was mixed with
80 μl HCl (0.6 M), 320 μl of Tris-TBA (26 mM–
120 mM), vortexed, and then incubated at 80 °C for
10 min. The resulting coloured upper layer was mea-
sured at 532 nm. The TBARS concentration was
calculated by referring to the extinction coefficient
of TBARS (1.56×10
5
M
−1
cm
−1
) and expressed per
milligram of protein.
Superoxide dismutase
The activity was determined at 25 °C by measuring its
ability to inhibit the photoreduction of nitroblue tetrazo-
lium (NBT) to the blue formazan [8]. The assay was
performed in an aerobic m ixture consisting of PBS
(50 mM), methionine (13 mM), EDTA (0.1 mM) ribo-
flavine (2 μM), and NBT (75 μM). The activity was
expressed as units/milligram protein, 1 U being the
amount inhibiting the photoreduction of NBT by 50 %.
Catalase
The catalase (CAT) activity was determined by mea-
suring the decrease in absorbance at 240 nm for 2 min
at 25 °C of H
2
O
2
(final concentration 20 mM) accord-
ing to the metho d of Aebi [1]. CAT activity was
expressed as micromoles of H
2
O
2
destroyed/minute/
milligram protein at 25 °C.
Glutathione peroxidase
The GPx activity was assayed at 25 °C according to the
method of Flohé and Günzler [11]withsomemodifica-
tions. The activity was measured in 250 μl cellular
230 E. Bourogaa et al.

extract mixed with GSH (final concentration, 0.35 mM).
Reactions were started with the addition of H
2
O
2
(0.2 mM). After the reactions were stopped, 5,5′-dithio-
bis-2-nitrobenzoic acid was added, and the absorbance
was recorded at 412 nm. Such activity was expressed as
micromoles of GSH oxidized/minute/gram protein.
Histological examination
A portion of liver was processed by routine histology
procedure. After fixation in Bouin solution, pieces of
fixed tissue were embedded into paraffin, cut into
5 μm slices, a nd colored with hematoxylin–eosin.
For evaluation of histological alterations, these slides
were observed under light microscop e.
Electrophoresis and Western blotting
For Western blot analysis, liver tissues (1 g) were re-
duced in Laemmli sample buffer, sonicated, and boiled
for 3 min. Proteins were then resolved on sodium
dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to nitrocellulose mem-
brane (Hybond-C Super, Amersham Pharmacia Bio-
tech). The membrane was block ed for 1 h in TBS-
Tween 0.05 % (TBS-T) containing 5 % of milk.
Blocked membranes were then blotted with antibodies
anti-GSK3β (BD Transduction Laboratories) and anti-
phospho Ser9 GSK3 (Biosource International) over-
night at 4 °C, washed twice with TBS-T, and
incubated for 1 h with horseradish-peroxidase-coupled
secondary antibody (Cell Signalling). After three addi-
tional washes, detection was achieved with Pierce
Supersignal chemiluminescent substrate (Pierce).
Statistical analysis
The results are expressed as mean±SEM. The signif-
icance of the differences between the control and the
treatment was established by the Student’s t test for
independent samples (P<0.05).
Results
Characterization of H. scoparia extracts
The results of the preliminary phytochemical screen-
ing method are illustrated in Table 1. In fact, the
chemical tests performed in DE and ME extracts for
sterols, triterpenoids, carotenoids, tropolons, and qui-
nons were negative. However, the chemical test per-
formed for alkaloids was positive in ME and very
important in DE, while the chemical test for flavo-
noids was positive only for ME. Moreover, phenolic
contents of the plant extracts were evaluated using the
Folin–Ciocalteu reagent. Analysis shows the yield and
the phenolic content in H. scoparia extract, expressed
as milligram of gallic acid equivalents per gram of
extract. ME showed a highest amount of phenolic
compounds (58.82±0.082 mg/g; Table 2).
The antioxidant activities of plant extracts were
evaluated by its ability to scavenge DPPH free radi-
cals. ME showed a scavenging activity with an impor-
tant decr ease of D PPH free ra dicals versus the
scavenging activity of α-tocopherol and ascorbic acid
(Table 3). In addition, AAC are summarized in Table 3
where the results showed that ME and BHT ha s a
comparable antioxidant activity.
Hematological parameters
The blood parameter levels such as RBC, WBC, and
hematocrit are represented in Table 4. Indeed, ethanol
administration increased WBC levels to 4.12±0.11
[from 3.25±0.12 in control groups (p<0.05)]. WBC
levels were significantly decreased (p<0.05) in animals
treated with ME (3.37±0.124).
Liver function
Ethanol administration affected biochemical markers
(AST, ALT, and ALP) of liver function. As shown in
Table 5, serum AST, ALT, and ALP levels were
Table 1 Chemical groups
present in the extracts from leaves
of Hammada scoparia
(−) No reaction, (+) positive
reaction, (++) important reaction
Sterols/
Triterpenoids
Carotenoids/
Triterpenoids
Tropolons Quinones Flavonoids Alkaloids
HE + −−−−−
DE −−−−−++
ME −−−−++
Hepatoprotective potential of H. scoparia 231

enhanced, compared to control group, in chronically
ethanol-treated rats (59, 55, and 71 %, respectively).
When animals were treated with H. scoparia extracts
(DE and ME), the activities of aminotransferases were
practically restored and reached the normal values of
control rats. Particularly, the beneficial effect of the
ME treatment on the biochemical parameters of liver
injury was highly significant (P<0.001).
Evaluation of lipid peroxidation and antioxidant
enzymes (CAT, SOD, and GPx)
Liver TBARS levels, a lipid peroxidation marker,
increased significantly after alcohol treatment by a
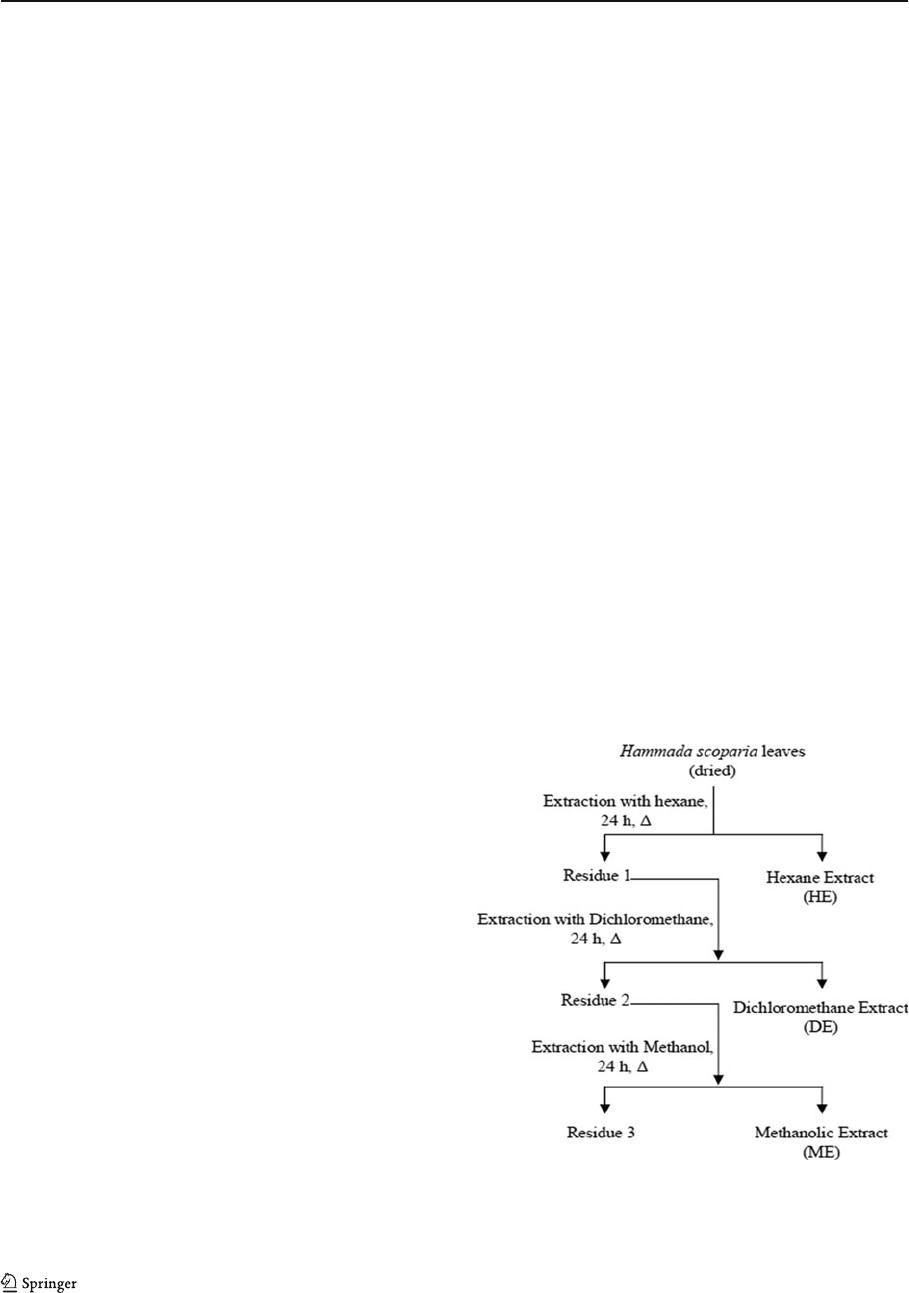
factor of 2.8 compared with controls (Fig. 2a). This
effect was completely inhibited in the presence of H.
scoparia extracts, especially in ME extract enriched in
flavonoids (Table 1).
To evaluate the ability of ME to prevent ROS-
induced oxidative stress, SOD, CAT, and GPx activi-
ties were meas ured in liver homogenates. Figure 2b–d
shows that rats treated with ethanol exhibited a
marked decrease in the activities of SOD, CAT, and
GPx. Treatment with H. scoparia extracts (HE, DE,
and ME) could not repair the reduction in SOD and
CAT activities, while GPx activity was restored after
H. scoparia methanolic extract treatment.
Histological examination showed a protective effect
of ME
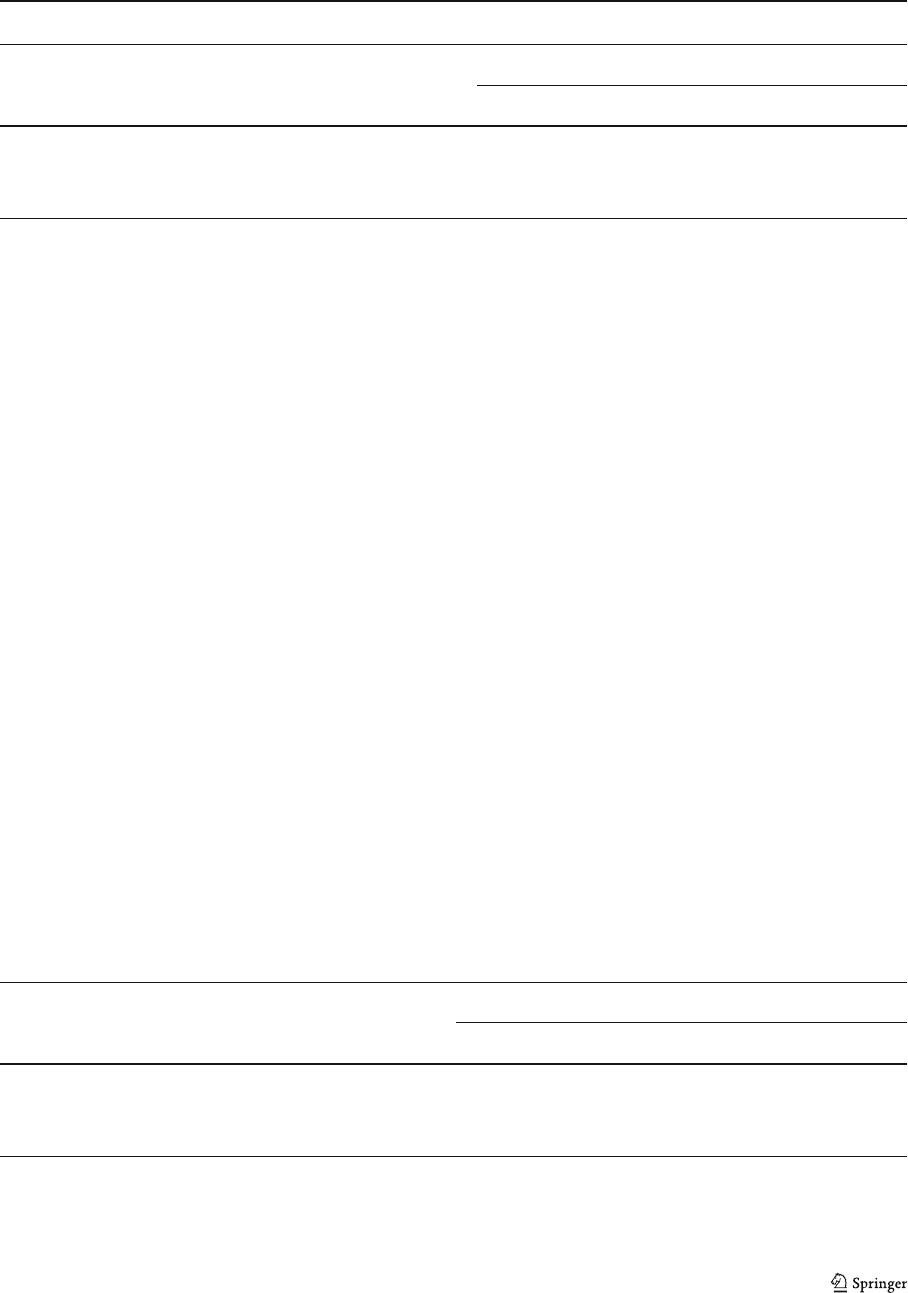
The liver sections of ethanol-intoxicated rats proved
massive ballooning degeneration and cytoplasmic
vacuolization (Fig. 3b) compared to a normal histolo-
gy in control livers (Fig. 3a). The hepatocellular dam-
age was slightly reduced in ethanol-fed rats treated
with HE or DE (Fig. 3c, d). Nevertheless, the histo-
logical architecture of liver sections of the rats treated
with methanolic extract demonstrated prominent re-
covery in the form of maintained hepatic histoarchi-
tecture (Fig. 3e), such as reduced cytoplasmic
vacuolization and ballooning degeneration.
Glycogen synthase kinase
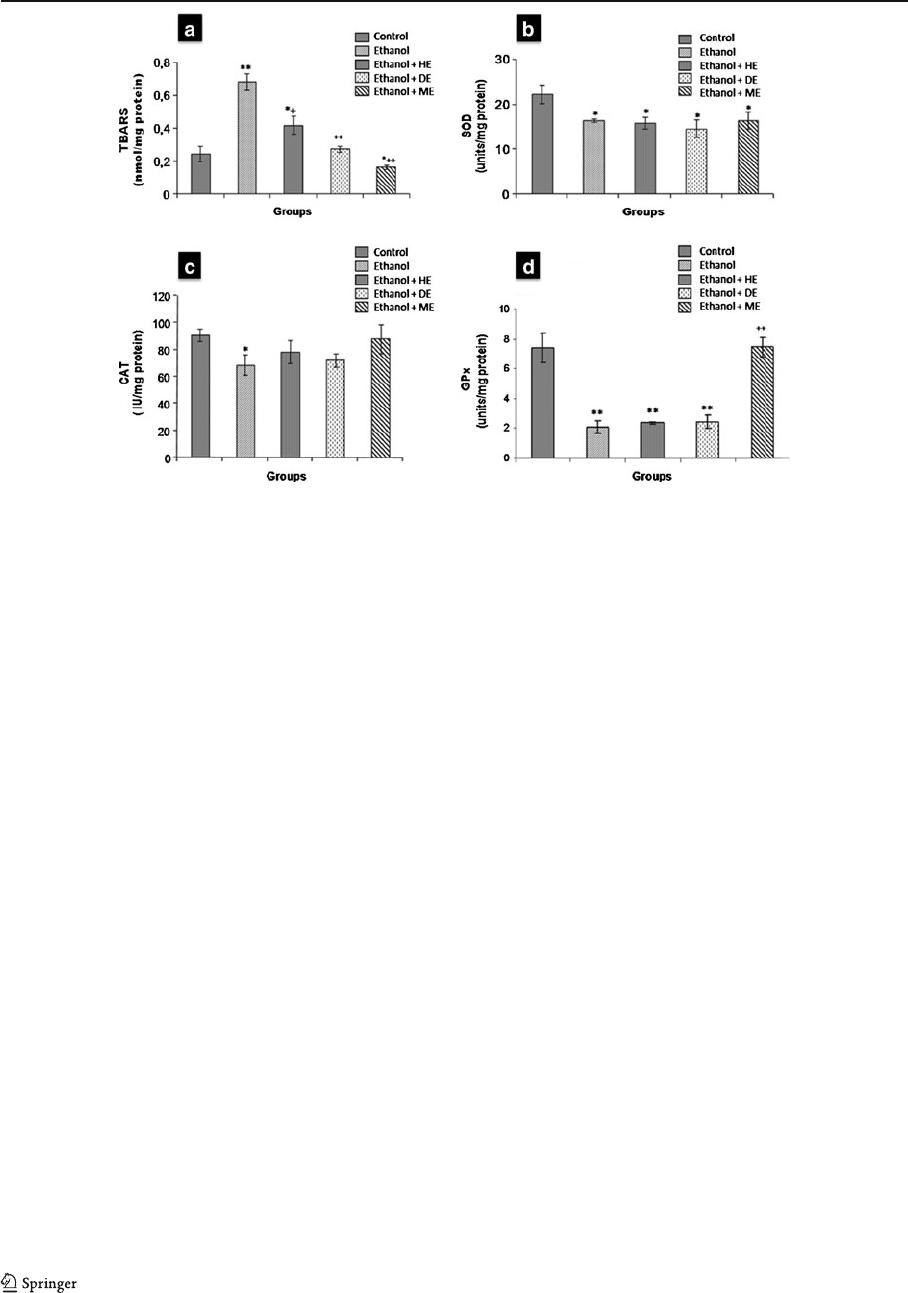
Immunoblot analysis was used to examine the conse-
quences of chronic ethanol consumption on total
GSK-3β in liver h omogenate. The data presented in
Fig. 4 show that GSK-3β was overexpressed after
ethanol treatment. However, ethanol was not able to
induce such effect in rats r eceiving H. scoparia
extracts (HE, DE, and ME). We suggest that ethanol
activates GSK-3β, which was demonstrated using the
anti-phospho Ser
9
GSK-3β anti body (Fig. 4).
Discussion
The liver is the major site of xenobiotic metabolism
and excretion. This organ accounts for 90 % of alcohol
metabolism. Indeed, it is the most adversely affected
organ after an excessive consumption of alcohol. The
alcohol absorbed from the intestinal tract gains access
first and foremost to the liver, resulting in a variety of
liver ailments. Thus, liver diseases remain one of the
serious health problems due to alcohol abuse [6].
In the present study, the hepatoprote ctive effect of
hexane, dichlorom ethane, and methanolic extracts of
H. scoparia leaves was evaluated in alcoholic rat
model a nd find out the therapeutically efficacious
extract. An attempt was made to find out the correlation
Table 2 The total phenolic content and antioxidant activities in
vitro (2,2-Diphenyl-1-picrylhydrazyl and β-carotene bleaching
method)
Extract TPC (mg of gallic
acid equivalents/g)
HE (hexane extract) 9.76±0.030
DE (dichloromethane extract) 34.75±0.085
ME (methanol extract) 58.82±0.082
Total polyphenolic content in the extracts of H. scoparia leaves
expressed in terms of gallic acid
Table 3 The total phenolic content and antioxidant activities in
vitro (2,2-Diphenyl-1-picrylhydrazyl and β-carotene bleaching
method)
Extract IC
50
(μg/ml) AAC
HE (hexan extract) 23.6 485
DE (dicloromethan extract) 6.3 606
ME (methanolic extract) 2.6 666
α-Tocopherol (antioxidant reference) 5.5 805
Ascorbic acid (antioxidant reference) 3.5 –
BHT (antioxidant reference) – 753
DPPH-scavenging activity of plant extracts (IC
50
) and antioxi-
dant assay using the β-carotene bleaching method (AAC anti-
oxidant activity coefficient)
232 E. Bourogaa et al.
between antioxidant and hepatoprotective activity. In
fact, ethanol is being used extensively to investigate
hepatoprotective activity on various experimental ani-
mals. Its administration induces oxidative stress either
by enhancing the production of ROS and decreasing the
level of endogenous antioxidants [13]. Moreover, etha-
nol is readily absorbed from the gastrointestinal tract.
Actually, only 2–10 % is eliminated through the kidneys
and the lungs, the rest is oxidized in the body, first and
foremost in the liver [17]. A major defense mechanism
involves the antioxidant enzymes, including SOD, CAT,
and GPx, which convert active oxygen molecules into
nontoxic compounds.
Besides, excessive use of alcohol induces lipid
peroxidation (when free radicals are formed), damages
the liver cells membranes and o rganelles, and causes
the swelling and necrosis of hepatocytes, resulting in
the release of cytosolic enzymes such as AST, ALT,
and ALP into the circulating blood [32].
The ethanol oxidizing system takes place in micro-
somes, involving an ethanol-inducible cytochrome
P450 (2E1). After chronic ethanol consumption, there
is a four- to tenfold induction of P450 (2E1) expres-
sion, associated not only with increased acetaldehyde
generation but a lso with the production of oxygen
radicals that promote lipid peroxidation [18, 22, 29].
In this view, the reduction in AST, ALT, and ALP
activities by metha nolic extracts is an indication of the
stabilization of plasma membrane as well as a repair of
hepatic tissue damage caused by ethanol. This effect is
in agreement with the commonly accepted view that
serum activities of aminotransferases return to normal
with the healing of hepatic parenchyma and regenera-
tion of hepatocytes [30]. Alkaline phosphatase is the
prototype of these enzymes that reflects the patholog-
ical alteration in biliary flow.
Thus, the administration of methanolic extract of
leaves reveal ed hepatoprotective activity of H. scopa-
ria leaves against the toxic effect of ethanol, which
was also supported by histopathological studies.
Reduced lipid peroxidation was revealed by signif-
icant decrease in TBARS level in groups treated by
extracts, especially in ME extract enriched in flavo-
noids. Furthermore, histopatholog ical studies under
light microsco pe confirm th e preventive effect of
metanolic extract agains t ethanol induced liver dam-
age as shown by the decrease in the severity of toxic
effects of ethanol (vacuolization and ballooning). This
Table 4 Blood parameters of rat after chronic ethanol administration treated with or without H. scoparia extracts (HE, DE, and ME)
Parameter Control Effect of EtOH Effect of EtOH+H. scoparia extracts
HE DE ME
RBC (10
6
/mm
3
) 6.83±1.603 6.91±1.431 6.79±1.714 6.53±1.226 6.69±1.492
WBC (10
3
/mm
3
) 3.25±0.126 4.12±0.117* 3.47±0.167** 3.28±0.212** 3.37±0.124**
Hematocrit 0.414±0.01 0.408±0.012 0.395±0.014 0.420±0.016 0.418±0.008
Data represent mean±SEM of six rats. Symbols represent statistical significance
*P<0.05, when compared with control group; **P <0.05, when compared with ethanol group
Table 5 Biochemical indicators of liver function in serum of rats after chronic ethanol administration injected with or without H.
scoparia extracts
Parameter Control Effect of EtOH Effect of EtOH+H. scoparia extracts
HE DE ME
AST (IU/L) 114.50±3.12 192.16±5.49* 185.33±4.58* 170.33±6.91*
,
** 122.83±4.10***
ALT (IU/L) 53.66 ±2.80 96.50±2.66* 98.83±4.11* 84.83±3.45* 46.66±2.49***
ALP (IU/L) 186.16±4.62 260.83±4.49* 254.83±3.36* 249.33±3.12* 191.00±4.27***
Data represent mean±SEM of six rats. Symbols represent statistical significance
AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosph atase
*P<0.05 when compared with control group; **P<0.05, ***P<0.001, when compared with ethanol group
Hepatoprotective potential of H. scoparia 233
severity was much less when seen in sections treated
with ME (200 mg/kg) and was comparable to control.
The above results demonstrate that hepatoprotective
effect of plant extract may be due to inhibitory effect
on free radical formation.
In addition, to maintain redox homeostasis, aerobic
cells have developed an antioxidant system implicat-
ing phase II detoxification genes [23]. These genes
involve NAD(P)H, glutathione S-transferase, glutathi-
one peroxidase, and heme oxygenase-1. The transcrip-
tional factor nuclear factor E2-related factor 2 (Nrf2)
regulates the expression of antioxidant phase II
genes and contributes to the preserv ation of redox
homeostasis and cell viability in response to oxi-
dants damage. As a matter of fact, Nrf2 regulates
the expression of glutathione peroxidase and pro-
tects against hydrogen peroxide-induced glutathione
depletion [24]. When cells wer e exposed to reactive
oxygen species, Nrf2 is released from the cytoplas-
mic complex with keap1, leading to its translocation
to the nucleus, where Nrf2 activates transcription of
antioxidant phase II genes [20], such as glutathione
peroxidase. Salazar has shown that active GSK-3β
phosphorylates Nrf2 resulting in its exclusion from
the nucleus [24].
Superoxide dismutase, catalase, and glutathione
peroxida se ac tivities decreas ed sig nificantly in
ethanol-treated rats, which is in agreement with
previous data demonstrating a decrease in antioxi-
dant defense activities in rat liver exposed to eth-
anol [31]. Treatment with plant extracts showed
that only GPx activity was corrected in the group
receiving ME. However, SOD and CAT activities
did not move, compared to EtOH group, following
treatment with plant extract (HE, DE, and ME),
reflecting that the protective effect of ME involves
the GPx pathway without adjusting SOD and CAT
activities. Therewith, the decrease in TBARS lev-
els in animals receiving ME with respect to the
controls is strongly related to the standardization
ofGPxactivityinliver.
The significant increase in GPx content in the
liver, after ME treatment, suggests an antioxidant
activity of H. scoparia leaves extracts. Thus, it can
be concluded that a possible mechanism of hepato-
protective activity of H. scoparia leaves may be
Fig. 2 Effect of ethanol-treatment (for 4 weeks) with or without
H. scoparia extracts (HE, DE, and ME) on lipid peroxidation and
antioxidant enzymes activities in liver. a Thiobarbituric acid-
reacting substances (TBARS); b superoxide dismutase activity,
SOD represents the amount of enzyme that inhibits the oxidation
ofNBTby50%/mgprotein;c catalase activity (CAT; μmol H
2
O
2
/
min/mg protein); d glutathione peroxidase activity (GPx; μmol
GSH/min/mg protein). Values are the mean±SEM (n0 6. **P<
0.01, significantly different from control group; *P<0.05, signif-
icantly different from control group.
++
P<0.01, significantly dif-
ferent from ethanol-treated group;
+
P<0.05, significantly different
from ethanol-treated group)
234 E. Bourogaa et al.
due to the inactivation of GSK-3β after plant ex-
tract (ME) treatment. Once GSK-3β is inactivated,
Nrf2 is not phosphorylated, not excluded from the
nucleus, and therefore, they induce the expression
Fig. 4 Hepatic immunoblot
analysis of GSK-3β and
phospho-Serine
9
GSK-3β
(P-Ser9 GSK-3β): Ethanol
treatment induces GSK-3β
expression. This increase
was bloqued in liver of rats
receiving H. scoparia
extracts (HE, DE, and ME).
GSK-3β was immunopreci-
pitated with monoclonal anti-
GSK-3β and Ser
9
GSK-3β
phosphorylation was deter-
mined by immunoblot analy-
sis with phospho-specific
antibody
Fig. 3 a Liver sections of
normal control rats showing
normal hepatic cells. b Liver
section of ethanol treated
rats showing: vacuolization
and ballooning degenera-
tion. c– e Liver sections of
ethanol-fed rats treated with
HE, DE, and ME, respec-
tively (V vacuolization, B
ballooning) (HE, ×200)
Hepatoprotective potential of H. scoparia 235
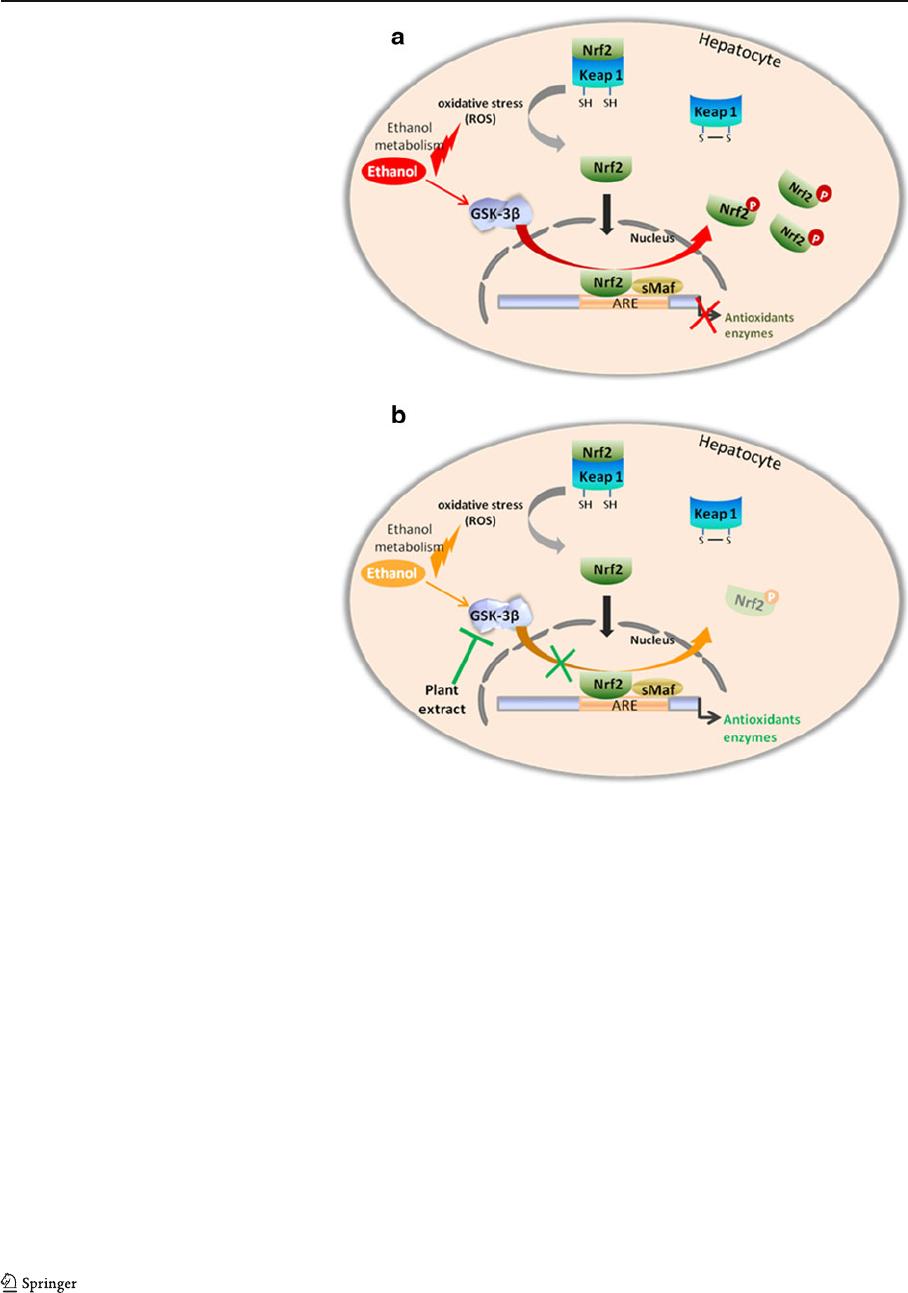
of glutathione peroxidase (Fig. 5). This antioxidant
activity may be attributable to the presence of phe-
nolic compounds in methanolic extract, which pre-
sented a potent antiradical activity in comparison
with ascorbic acid.
In fact, we previously isolated from H. scoparia
polar extract some phenolic alkaloids (N-methylisosal-
soline, 1-methylisosalsolinol, and dopamine) and fla-
vonoids (quercetin, isorhamnetin, and quercetin 3-O-
robinobioside) [14].
Our results confirm the relationship between the
glycogen synthase kinase and glutathione peroxi-
dase. Immunoblot analysis showed an activation of
GSK-3β in liver of ethanol treated animals. These
data were in agreement with those of Shin et al.
[27], who showed that H
2
O
2
activates GSK-3β.
The activation of GSK-3β mediates phosphorylation
of Nrf2 and its exclusion from the nucleus, which
was marked by a decrease in GPx activity
(Fig. 5a). GPx gene silencing was prevented by
methanolic extract of H. scoparia leaves adminis-
tered simultaneously with ethanol treatment. This
could be explained by the inactivation of GSK-3 β
after treatment with the extract.
Conclusion
The present study has proved that the hepatoprotective
action of methanolic extract of H. scoparia, the most
effective among the ones tested, may be due to its
antioxidant activity as indicated by the protection
against inc reased lipid peroxidation and maintained
GPx activity.
Fig. 5 Possible mechanisms
for the antioxidative effect
of methanolic extract in liver
cells. a Inactivation of
antioxidant enzymes after
ethanol administration. b
Protective ef fect of methanolic
extract
236 E. Bourogaa et al.
