
CLINICAL INVESTIGATION INTERVENTIONAL ONCOLOGY
Short-term Safety and Quality of Life Outcomes Following
Radioembolization in Primary and Secondary Liver Tumours:
a Multi-centre Analysis of 200 Patients in France
Romaric Loffroy
1
•
Maxime Ronot
2
•
Michel Greget
3
•
Antoine Bouvier
4
•
Charles Mastier
5
•
Christian Sengel
6
•
Lambros Tselikas
7
•
Dirk Arnold
8
•
Geert Maleux
9
•
Jean-Pierre Pelage
10
•
Olivier Pellerin
11
•
Bora Peynircioglu
12
•
Bruno Sangro
13
•
Niklaus Schaefer
14
•
Marı
´
a Urda
´
niz
15
•
Nathalie Kaufmann
15
•
Jose
´
Ignacio Bilbao
16
•
Thomas Helmberger
17
•
Vale
´
rie Vilgrain
2
•
On behalf of the CIRT-
FR Principal Investigators
Received: 16 July 2020 / Accepted: 2 September 2020
Ó The Author(s) 2020
Abstract
Purpose Radioembolization has emerged as a treatment
modality for patients with primary and secondary liver
tumours. This observational study CIRT-FR (CIRSE Reg-
istry for SIR-Spheres Therapy in France) aims to evaluate
real-life clinical practice on all patients treated with
transarterial radioembolization (TARE) using SIR-Spheres
yttrium-90 resin microspheres in France. In this interim
analysis, safety and quality of life data are presented. Final
results of the study, including secondary effectiveness
outcomes, will be published later. Overall, CIRT-FR is
aiming to support French authorities in the decision making
on reimbursement considerations for this treatment.
Methods Data on patients enrolled in CIRT-FR from
August 2017 to October 2019 were analysed. The interim
analysis describes clinical practice, baseline characteristics,
safety (adverse events according to CTCTAE 4.03) and
quality of life (according to EORTC QLQ C30 and HCC
module) aspects after TARE.
Results This cohort included 200 patients with hepatocel-
lular carcinoma (114), metastatic colorectal cancer
(mCRC; 38) and intrahepatic cholangiocarcinoma (33)
amongst others (15). TARE was predominantly assigned as
a palliative treatment (79%). 12% of patients experienced
at least one adverse event in the 30 days following treat-
ment; 30-day mortality was 1%. Overall, global health
Electronic supplementary material The online version of this
article (https://doi.org/10.1007/s00270-020-02643-x) contains sup-
plementary material, which is available to authorized users.
& Nathalie Kaufmann
1
Department of Vascular and Interventional Radiology,
Image-Guided Therapy Center, CHU Dijon Bourgogne,
Franc¸ois-Mitterrand University Hospital, 14 Rue Gaffarel,
21000 Dijon, France
2
Department of Radiology, Universite
´
de Paris, Ho
ˆ
pital
Beaujon APHP, and CRI, INSERM 1149, Paris, France
3
Service d’Imagerie interventionnelle, Ho
ˆ
pitaux Universitaires
de Strasbourg, 1 Avenue Moliere, 67000 Strasbourg, France
4
Department of Radiology, Angers University Hospital, 4 Rue
Larrey, 49933 Angers, France
5
Interventional Radiology, Centre Le
´
on Be
´
rard, 28 Prom. Le
´
a
Et Napole
´
on Bullukian, 69008 Lyon, France
6
Interventional Radiology, Centre Hospitalier Universitaire de
Grenoble, Boulevard de la Chantourne, 38100 Grenoble,
France
7
Interventional Radiology, De
´
partement d’anesthe
´
sie
Chirurgie et Interventionel (DACI), Gustave Roussy,
Universite
´
Paris-Saclay, Villejuif, France
8
Oncology and Hematology, Asklepios Tumorzentrum
Hamburg, AK Altona, Paul-Ehrlich-Str. 1, 22763 Hamburg,
Germany
9
Radiology, Universitair Ziekenhuis Leuven, Herestraat 49,
3000 Leuven, Belgium
10
Department of Diagnostic Imaging and Interventional
Radiology, Caen University and Medical Center, avenue de
la Cote de Nacre, 14033 Caen, France
11
Interventional Radiology Department, Ho
ˆ
pital Europe
´
en
Georges Pompidou, 20 rue Leblanc, 75015 Paris, France
12
Department of Radiology, School of Medicine, Hacettepe
University, Sihhiye Campus, 06100 Ankara, Turkey
13
Clı
´
nica Universidad de Navarra, IDISNA and CIBEREHD,
Liver Unit, Avda. Pio XII 36, 31008 Pamplona, Spain
123
Cardiovasc Intervent Radiol
https://doi.org/10.1007/s00270-020-02643-x

score remained stable between baseline (66.7%), treatment
(62.5%) and the first follow-up (66.7%).
Conclusion This interim analysis demonstrates that data
regarding safety and quality of life generated by ran-
domised-controlled trials is reflected when assessing the
real-world application of TARE.
Trial Registration Clinical Trials.gov NCT03256994.
Keywords Radioembolization Transarterial
radioembolization Yttrium-90 SIR-spheres
Interim analysis SIRT
Introduction
Transarterial radioembolization (TARE), also known as
selective internal radiation therapy (SIRT), with SIR-
Spheres Y-90 resin microspheres, is an endovascular pro-
cedure included within the interventional oncologic arma-
mentarium to treat primary and secondary liver tumours
[1–7].
CIRT-FR (CIRSE Registry for SIR-Spheres Therapy in
France; NCT03256994) was developed as a France-only
adaptation of the pan-European, prospective observational
study CIRT (NCT02305459) [8]. While CIRT [9] had only
collected data from one French hospital, CIRT-FR was
open to all French centres and designed to exhaustively
include as many patients in France as possible. Under the
condition that the present study was conducted in order to
collect data on the real-life clinical application of TARE
with SIR-SpheresÒ in France, reimbursement for liver-
only metastatic colorectal cancer (mCRC) was granted for
5 years by the French national health authorities (Haute
Autorite
´
de Sante
´
, HAS) in March 2017.
1
In March 2019,
based on the results of the phase III randomized controlled
trials SARAH [10] and SIRveNIB [11], HAS extended
reimbursement for patients with intermediate or advanced
hepatocellular carcinoma (HCC
2
). Final data from this
observational study will support French authorities in the
decision making on reimbursement considerations for
TARE beyond 2022.
This publication is the 200-patient interim analysis of
the prospective, post-market, observational study CIRT-
FR, with the objective to report on patient characteristics,
treatment details as well as safety and health-related quality
of life.
The primary objective was to observe the real-life
clinical application of TARE with SIR-Spheres Y-90 resin
microspheres in the context of the patients’ continuum of
care (please refer to Table 1). Secondary objectives were to
assess safety, toxicity, quality of life, technical considera-
tions and diagnosis and treatment-related considerations,
such as intention of treatment, prior and post-TARE
interventional procedures and/or systemic therapy.
Methods
Study Design/Setting
Patients for this 200-patient interim analysis were enrolled
from 11 centres between August 2017 and October 2019,
and follow-up data were collected until June 2020. All
hospitals in France that had performed TARE with SIR-
Spheres or were preparing to perform their first treatment
were invited to participate. Hence, 31 sites were invited to
participate, of which 4 declined participation, 4 were in the
contracting process at the time of this analysis and 22 sites
participated. Data were monitored by CIRSE remotely
every 3 months to verify data quality and completion, and
to identify any issues with patient inclusion and data col-
lection. This study was performed in accordance with the
Declaration of Helsinki and Good Clinical Practice
Guidelines and was approved by the CPP SUD-EST II
French Ethics Committee (N° ID-RCB: 2017-A01003-50).
Since this study is an offspring of the European observa-
tional study CIRT [9] and closely corresponds to its
methodology, please refer to the published methodology of
CIRT for a more detailed description of methods and
outcome measures [8].
14
Service de me
´
decine nucle
´
aire Et Imagerie mole
´
culaire,
Centre Hospitalier Universitaire de Lausanne, Rue du
Bugnon 46, CH-1011 Lausanne, Switzerland
15
Clinical Research Department, Cardiovascular and
Interventional Radiological Society of Europe, Neutorgasse
9, 1010 Vienna, Austria
16
Interventional Radiology, Clı
´
nica Universidad de Navarra,
Avenida Pio XII, no 36, 31008 Pamplona, Spain
17
Department of Radiology, Neuroradiology and Minimal-
Invasive Therapy, Klinikum Bogenhausen, Englschalkinger
Str. 77, 81925 Munich, Germany
1
HAS (2015). Avis de la CNEDiMTS sur SIR-Spheres. Retrieved
March 2, 2017, from https://www.has-sante.fr/portail/jcms/c_
2023879/fr/sir-spheres
2
HAS (2018). Avis de la CNEDiMTS sur SIR-Spheres. Retrieved
October 7, 2019, from https://www.has-sante.fr/jcms/c_2896412/fr/
sir-spheres
R. Loffroy et al.: Short-term Safety and Quality of Life Outcomes...
123
Patients
Patients were required to be at least 18 years old and to
have primary or metastatic liver cancer intended to be
treated with TARE (using SIR-Spheres) and to have signed
an informed consent form. There were no further specified
inclusion or exclusion criteria. Since the aim of the study
was to capture all patients treated with SIR-Spheres in
France, patients included in other trials were permitted to
be enrolled. As a result, some intrahepatic cholangiocar-
cinoma patients of the present study were treated within the
SIRCCA trial (NCT 02807181), but there were no patients
in common with the European CIRT registry [9]. All
aspects related to treatment and follow-up were performed
at the discretion of the treating physicians. It was recom-
mended to perform follow-ups every 3 months, for a period
of at least 24 months.
Outcomes and Data Sources
The primary outcome was defined as the real-life clinical
application of TARE in order to assess at which stage of
the cancer continuum of care the therapy was utilised.
Secondary endpoints covered in this interim analysis were
safety data and health-related quality of life (HRQOL)
[12, 13]. For safety, adverse events (AE) and abnormal
laboratory findings were graded according to the Cancer
Institute’s Common Terminology Criteria for Adverse
Events (CTCAE) v4.03. Adverse events were classified
into three main groups; peri-procedural AE (on the same
day of the treatment), AE within 30 days after treatment
and AE after more than 30 days after treatment.
HRQOL was assessed using the EORTC QLQ-C30
questionnaire, as well as the QLQ-HCC18 Module for
patients with hepatocellular carcinoma (HCC) [14].
Questionnaires were to be filled before, within one week
after treatment and the first follow-up. Median first follow-
up was at 13 weeks (IQR = 3).
For the HRQOL analysis (according to version 3 of the
EORTC QLQ-C30 Scoring Manual (2001) and version 2 of
the EORTC QLQ-HCC18 Scoring Manual), only patients
with data available for all three timepoints (before treat-
ment, within one week after treatment, and the first follow-
up) were included, as a ‘‘per protocol’’ analysis. Scores for
global health, functionality and symptoms were calculated
by linearly transforming the mean of the respective raw
scores on a scale of 0 to 100. For the global health and the
functional score, a high score indicated high health and for
the symptoms and the HCC18 Module a low score indi-
cates few symptoms and better quality of life.
Bias
In order to reduce patient selection bias, participation in the
observational study was contractually required to be
offered to all patients with primary or secondary liver
tumours treated with TARE using SIR-Spheres. Further-
more, in order to assess patient coverage, case logs listing
the number of patients treated versus number of patients
actually enrolled in the study at each centre were collected
quarterly from each of the 22 participating centres (please
refer to supplementary Table 1 for more information).
Statistical Methods
Baseline, treatment data and the secondary endpoint of
safety were presented using summaries and descriptive
statistics. For continuous data median, minimum and
maximum were shown. To analyse categorical data, counts
and percentages were used.
Table 1 Treatment intent (Primary outcome measurement)
Primary endpoint HCC
n = 114 (%)
ICC
n = 33 (%)
mCRC
n = 38 (%)
(A) First-line TARE treatment with or without concomitant systemic therapy 43 (37.7) 18 (54.5) –
(B) Second or subsequent line TARE treatment with or without concomitant systemic therapy after
previous first-line systemic therapy, including salvage therapy when no other systemic therapies
used alone are likely to be efficacious
7 (6.1) 8 (24.2) 20 (52.6)
(C) TARE treatment with or without concomitant systemic therapy after previous interventional
liver-directed procedures or liver surgery
50 (43.8) 1 (3.0) 2 (5.2)
(D) Addition of TARE to systemic therapy (any line) or to any other treatment (e.g. ablation)
intended as part of a multimodal curative therapy with any of the following objectives:
resectability and/or ablative therapy and/or transplantation
6 (5.3) 3 (9.1) 2 (5.2)
(E) Treatment with TARE in patients intolerant of chemotherapy or patients considered not
suitable for systemic therapy
2 (1.8) 1 (3.0) –
(F) Other 6 (5.3) 2 (6.1) 14 (36.8)
R. Loffroy et al.: Short-term Safety and Quality of Life Outcomes...
123

Table 2 Patient demographics and treatments before and after TARE
Patient demographics n (%)
Male 140 (70)
Age (median) (19, 92) 66
Disease characteristics
Primary liver cancer 151 (75.5)
Secondary/metastatic liver cancer 49 (24.5)
Primary tumours
HCC 114 (75.5)
ICC 33 (21.8)
Other 4 (2.7)
Metastatic liver cancer
CRC 38 (77.5)
NET 5 (10.2)
Gastric cancer 1 (2)
Lung cancer 1 (2)
Other (Prostate cancer, missing) 4 (8.2)
Prior hepatic procedures HCC n = 114 (%) ICC n = 33 (%) mCRC n = 38 (%)
Yes (subjects with at least one prior hepatic procedure) 61 (53.5) 2 (6.1) 18 (47.4)
No 53 (46.5) 31 (93.9) 20 (52.6)
Surgery HCC n = 16 (14.0) ICC n = 1 (3.0) mCRC n = 9 (23.7)
Liver surgery 13 (11.4) 1 (3.0) 9 (23.7)
Open 9 (7.9) 1 (3.0) 4 (10.5)
Laparoscopy 4 (3.5) – 2 (5.3)
Liver transplant 3 (2.6) – –
Ablation HCC n = 17 (14.9) ICC n = 0 (0) mCRC n = 4 (10.5)
Radiofrequency Ablation 9 (7.9) – 2 (5.3)
Microwave Ablation 10 (8.8) – 1 (2.6)
Intra-arterial treatments HCC n = 51 (44.7) ICC n = 1 (3.0) mCRC n = 4 (10.5)
Chemoembolization (TACE) 47 (41.2) – 2 (5.3)
Conventional TACE 45 (39.5) – 1 (2.6)
Drug-eluting TACE 2 (1.8) – 1 (2.6)
Hepatic-arterialchemotherapy – – 2 (5.3)
Bland embolization 1 (0.9) – –
Vascular other 2 (1.8) 1 (3.0) –
Radiation therapy (EBRT, Other) HCC n = 1 (0.9) ICC n = 0 (0) mCRC n = 0 (0)
Other (cyberknife) 1 (0.9) – –
Prior systemic chemotherapy HCC n = 16 (14) ICC n = 13 (40) mCRC n = 34 (90)
Hepatic Procedures after TARE HCC n = 114 (%) ICC n = 33 (%) mCRC n = 38 (%)
Yes (Subjects with at least one hepatic procedure after TARE) 24 (21.0) 3 (9.1) 2 (5.3)
No 82 (71.9) 28 (84.8) 31 (81.6)
Unknown/data not available 8 (7.1) 2 (6.1) 5 (13.1)
R. Loffroy et al.: Short-term Safety and Quality of Life Outcomes...
123

HRQOL data were plotted using RStudio under R 3.6.1.
For both questionnaires, changes of more than 10 points
were considered clinically relevant [15].
Results
Data Collection and Distribution of Patients
This interim report includes 200 patients, enrolled between
August 2017 and October 2019. Treatment data were
available for 98.5% and follow-up data for 93% of the
patients, respectively. 11 centres actively contributed
patient data, whereas another 11 centres performed no or
very few cases and did not provide patient data. Patient
numbers differed significantly between centres. In this
interim analysis, a vast majority of 81.5% (n = 163) were
treated at three sites; moreover, 54.5% (n = 109) of the
overall patient population was contributed by one site.
Median number of patients enrolled per site was 8.
With case logs collected quarterly from all 22 sites
involved in the study, it was determined that the achieved
coverage of all patients treated at participating sites was
84%. When disregarding one centre, the patient coverage
achieved was 91% (please refer to supplementary Table 1
for more information).
Patient Characteristics
Median age was 66 years (range 19–92), 140 (70%)
patients were male. Of all 200 patients, HCC represented
the majority (114, 57%) of cases, followed by mCRC (38,
19%) and intrahepatic cholangiocarcinoma (ICC; 33,
16.5%). Smaller cohorts were neuroendocrine tumour
(NET; 5, 2.5%), and other malignancies (10, 5%, see
Table 2).
More than half of the HCC patients (53.5%) underwent
liver-directed therapies before entering the study, mainly
conventional transarterial chemoembolization (TACE;
39.5%) and 14% prior systemic chemotherapy.
93.9% of ICC patients received no surgery or ablation
prior to TARE, but 40% received prior systemic
chemotherapy. While the majority (84.8%) of these
patients received no additional liver-directed treatment
after TARE, 66.7% received systemic chemotherapy
(Table 2).
In the mCRC cohort, 23.7% of patients received liver
surgery before TARE and 10.5% received tumour ablation.
Only 10% chemotherapy-naı
¨
ve patients were encountered
in the mCRC cohort, and 65.7% of patients received sys-
temic chemotherapy treatment after TARE.
Table 2 continued
Surgery HCC n = 4 (3.5) ICC n = 3 (9.1) mCRC n = 1 (2.7)
Liver surgery 1 (0.9) 3 (9.1) 1 (2.7)
Open 1 (0.9) 1 (3.0) 1 (2.7)
Laparoscopy – 2 (6.1) –
Liver transplant 3 (2.7) – –
Ablation HCC n = 4 (3.5) ICC n = 0 (0) mCRC n = 0 (0)
Radiofrequency ablation 1 (0.9) – –
Microwave ablation 2 (1.8) – –
Irreversible electroporation 1 (0.9) – –
Intra-arterial treatments HCC n = 22 (%) CC n = 0 (0) mCRC n = 1 (2.7)
Chemoembolization (TACE) 20 (17.5) – 1 (2.7)
Conventional TACE 16 (14.0) – –
Drug-eluting TACE 4 (3.5) – –
Hepatic-arterial chemotherapy 1 (0.9) – –
Bland embolization 1 (0.9) – –
Radiation therapy (TARE and EBRT) HCC n = 2 (1.8) I CC n = 0 (0) mCRC n = 0 (0)
Systemic chemotherapy after TARE HCCn = 28 (24.5) ICC n = 22 (66.7) mCRC n = 25 (65.7)
EBRT External beam radiation therapy
R. Loffroy et al.: Short-term Safety and Quality of Life Outcomes...
123

Table 3 Tumour location and
Tumour and Liver volumes
Liver tumour location HCC n = 114 (%) ICC n = 33 (%) mCRC n = 38 (%)
Left 22 (19.3) 6 (18.2) 3 (7.8)
Right 58 (50.9) 14 (42.4) 7 (18.4)
Bilobar 33 (28.9) 12 (36.4) 26 (68.4)
Unknown/data not available 1 (0.9) 1 (3.0) 2 (5.4)
Number of liver tumours
1 46 (40.3) 20 (60.6) 3 (7.9)
2 16 (14.0) 2 (6.1) 3 (7.9)
3 16 (14.0) 2 (6.1) 4 (10.5)
4 8 (7.0) – 2 (5.3)
5 4 (3.5) – 3 (7.9)
[ 5 7 (6.1) 3 (9.1) 8 (21.0)
Uncountable 15 (13.1) 5 (15.2) 11 (28.9)
Unknown/data not available 2 (1.8) 1 (2.9) 2 (5.3)
Liver and tumour volumes (mL) n = 195 Median (IQR)
Left lobe volume (mL) n = 55 638 (460.5)
Left tumour volume (mL) n = 35 60 (122.3)
Right lobe volume (mL) n = 54 1104.5 (623.4)
Right tumour volume (mL) n = 42 136 (341.5)
Total liver volume (mL) n = 129 1765 (910)
Total tumour volume (mL) n = 128 129.5 (363)
Tumour volume categories (mL) n = 197 (%)
1–50 41 (20.8)
51–100 37 (18.8)
101–200 30 (15.2)
201–500 43 (21.8)
501–1000 20 (10.2)
1001–2500 13 (6.6)
Unknown/data not available 13 (6.6)
Liver cirrhosis
Yes 81 (71.0) 4 (12.1) 1 (2.7)
Alcohol 20 (24.7) 3 (75) 1 (100)
Hepatitis B 2 (2.5) – –
Hepatitis C 19 (23.5) 1 (25) –
NAFLD 18 (22.2) – –
Alcohol ? Hepatitis C 6 (7.4) – –
Alcohol ? NAFLD 9 (11.1) – –
Other and unknown 7 (8.6) – –
No 33 (28.9) 28 (84.8) 35 (92.1)
Portal vein thrombosis (PVT) HCC n = 114 (%) ICC n = 33 (%) mCRC n = 38 (%)
Patent 79 (69.3) 28 (84.8) 33 (86.8)
Segmental 26 (22.8) 2 (6.1) 2 (5.3)
Lobar 5 (4.4) – –
Main 2 (1.8) 2 (6.1) –
Unknown/data not available 2 (1.8) 1 (3.0) 3 (7.9)
NAFLD non-alcoholic fatty liver disease
R. Loffroy et al.: Short-term Safety and Quality of Life Outcomes...
123

Table 4 Treatment procedure and administration
TARE treatment target Centre HCC n = 114 (%) ICC n = 33 (%) mCRC n = 38 (%)
Whole liver (single catheter) 1 1 (0.9) – –
2 – – 4 (10.5)
3 – – 1 (2.6)
6 – – 2 (5.3)
Whole liver (split administration/single session) 2 1 (0.9) 3 (9.1) 5 (13.2)
3 – 1 (3.0) –
6 – – 4 (10.5)
8 – – 1 (2.6)
Whole liver (sequential lobar/two sessions) 3 4 (3.5) – –
5 2 (1.8) – 1 (2.6)
Right lobe 1 45 (39.5) 15 (45.5) 5 (13.2)
2 4 (3.5) – 3 (7.9)
3 6 (5.3) – –
4 3 (2.6) 2 (6.1) –
5 2 (1.8) 1 (3.0) 2 (5.3)
6 2 (5.3)
7 2 (1.8) – 1 (2.6)
8 1 (0.9) – –
10 1 (0.9) – –
Left lobe 1 18 (15.8) 5 (15.2) 1 (2.6)
2 4 (3.5) – 1 (2.6)
3 5 (4.4) – 1 (2.6)
4 2 (1.8) 1 (3.0) –
5 – – 1 (2.6)
9 1 (0.9) – –
Segmental 1 4 (3.5) 3 (9.1) –
2 4 (3.5) 1 (3.0) 1 (2.6)
3 1 (0.9) – –
7 1 (0.9) – –
Unknown/data not available 1 1 (0.9) – –
7 – 1 (3.0) 2 (5.3)
11 1 (0.9) – –
Methodology for determining the dose
BSA 12 (10.5) 5 (15.2) 19 (50.0)
Modified BSA 19 (16.7) 1 (3.0) 5 (13.2)
Partition model 78 (68.4) 26 (78.8) 8 (21.0)
Other 2 (1.8) – –
Unknown/ data not available 3 (2.6) 1 (3.0) 6 (15.8)
Prescribed/delivered activity n = 231 (%)
Median prescribed activity whole (n = 126) 1 GBq
Unknown/data not available 3
Median prescribed activity right, GBq (n = 56) 1.2 GBq
Unknown/data not available 18
Median prescribed activity left, GBq (n = 40) 0.7 GBq
Unknown/data not available 34
R. Loffroy et al.: Short-term Safety and Quality of Life Outcomes...
123
Treatment Intent
Regarding the primary outcome of CIRT-FR, 37.7% of
HCC (n = 43/114) and 54.5% of ICC (n = 18/33) patients
underwent TARE with SIR-Spheres as a first-line treatment
(Table 1). 43.8% (n = 50/114) HCC, 3% (n = 1/33) ICC
and 5.2% (n = 2/38) mCRC patients received the treatment
after previous liver-directed interventional radiological
procedures or liver surgery in the absence of prior
chemotherapy and post-hepatic procedures. A total of
52.6% (n = 20/38) of mCRC patients, 24.2% (n = 8/33) of
ICC and 6.1% (n = 7/114) of HCC patients received TARE
treatment after previous first-line systemic therapy.
Tumour and Treatment-Related Characteristics
Please refer to Tables 3 and 4 for detailed information.
Safety
Peri-procedural adverse events were reported during
treatment for 8 (4%) patients, and 189 (94.5%) patients did
not present with peri-procedural adverse events (see
Table 5).
Within 30 days following treatment, 24 (12%) of
patients experienced at least one AE and a total of 42 AE,
with 82% of graded AEs reported to be mild to moderate
(i.e. grade 1 or 2). Six grade 3 AEs in three patients (GI
ulceration, radioembolization induced liver disease
(REILD), jaundice, oedema and dyspnoea) were reported.
No AE grade 4 or 5 was reported during the first 30 days.
Overall, 122 (61%) patients experienced at least one AE
more than 30 days following treatment and a total of 382
AE, with 83% of graded AEs being reported as moderate.
36 (9.4%) AEs in 28 (14%) patients were grade 3 or 4, and
no AEs grade 5 was reported. 168 (44%) AEs were not
graded. AEs of special relevance or interest for TARE are
listed in Table 5 together with commonly reported AEs.
The remaining reported AEs are categorised in Table 5,
section ‘’Less common AEs’’. No radiation induced
pneumonitis, cholecystitis or pancreatitis were reported in
the cohort.
Quality of Life
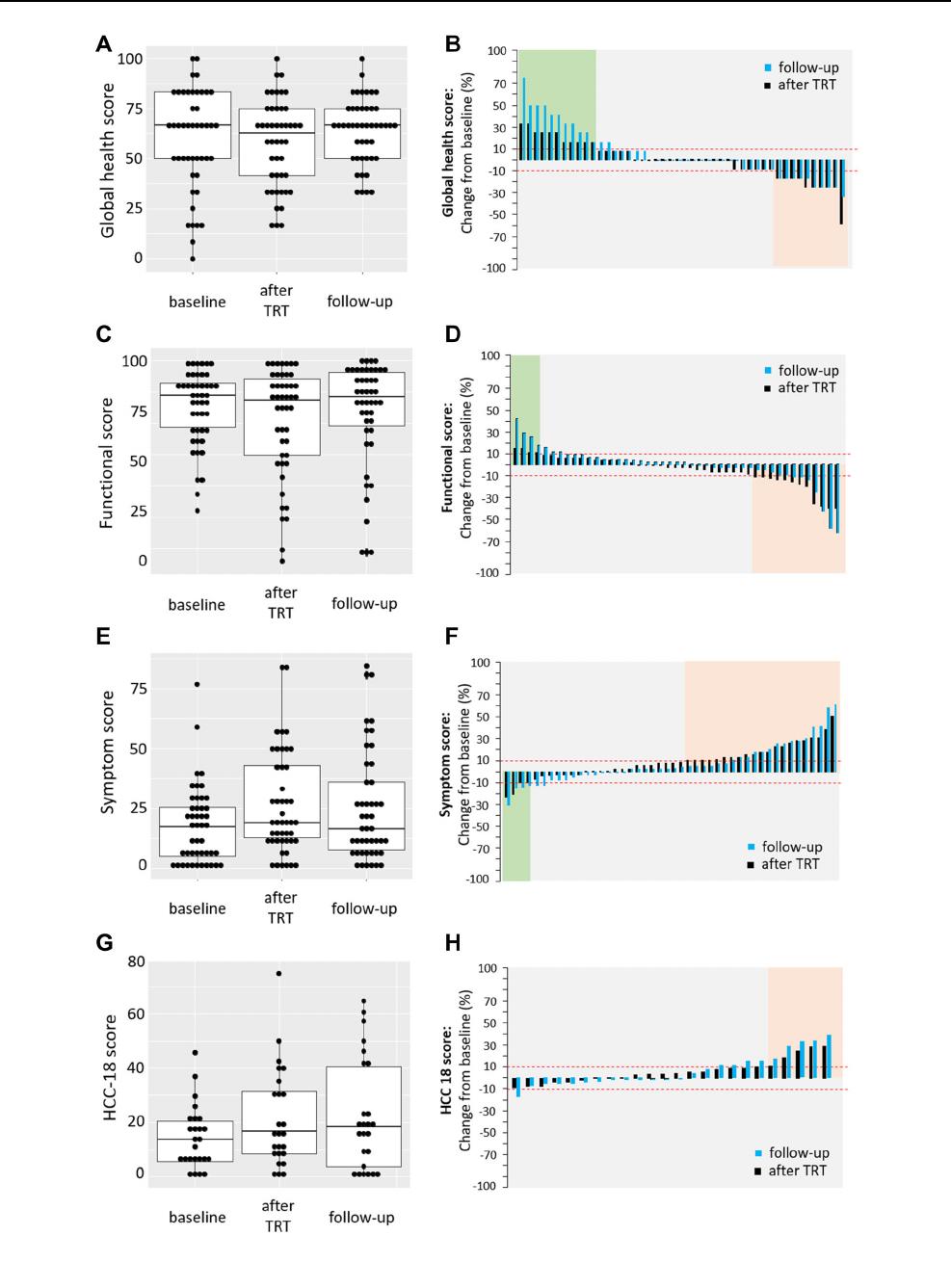
Health-related quality of life was assessed using the
EORTC QLQ-C30 questionnaire at baseline (range 99 –
0 days before treatment), within one week after treatment
and on the first follow-up at an average of 13 weeks (range
5–19) following treatment for the 23% of patients that had
questionnaires available for all three timepoints. Fig-
ure 1A, C and E shows that the median HRQOL at baseline
compared to after treatment and the first follow-up remains
stable for the global health score (66.7% vs. 62.5% and
66.7%), for the functional score (86.2% vs. 84.4% and
83.3%) and for symptom score (17.9% vs. 20.5% and
15.4%).
At a patient level, when comparing baseline to after
treatment, improvement indicated by an increase in more
than 10 points was seen in 23.9% for global health and
8.6% for functional and a decline by more than 10 points in
21.7 and 26%, respectively (Fig. 1B, D, black colour). A
decline in symptoms and thus improvement of HRQOL
was seen in 8.6% vs 45.6% showing increased symptoms
(Fig. 1F, black colour). The number of patients with
increased or decreased HRQOL did not change by much
when comparing the first follow-up to baseline (Fig. 1B, D,
F light colour). Quality of life status was distributed evenly
across indications (data not shown).
With regards to the HCC18 Module, information was
provided for 25 (22%) HCC patients at baseline at an
average of 13 days before treatment (range 0 to 43),
immediately after treatment and on the first follow-up at an
average of 13 weeks (range 5.4 to 18.4) following
Table 4 continued
Prescribed/delivered activity n = 231 (%)
Delivered activity within 90% of the prescribed activity n = 197 (%)
Yes 188 (95.4)
No 3 (1.5)
Unknown/data not available 6 (3.0)
Number of administrations delivered n = 197 (%)
1 59 (29.9)
2 24 (12.2)
3 3 (1.5)
Unknown/data not available 111 (56.3)
BSA, body surface area; GBq, gigabecquerel
R. Loffroy et al.: Short-term Safety and Quality of Life Outcomes...
123

Table 5 Peri-interventional adverse events (AE) and AEs summary
Peri-interventional AEs \ 30 days [ 30 days
Overall AE
n = 9 (%)
AEs Grade 3 or 4
n = 6 (%)
Overall AE
n = 42 (%)
AEs Grade 3
n = 6 (%)
Overall AE
n = 382 (%)
AEs Grade 3 or 4
n = 36 (%)
Abdominal pain 4 (44.4) 4 (66.7) 2 (4.8) – 50 (13.1) 4 (11.1)
Fatigue – – 11 (26.2) – 91 (23.8) 8 (22.2)
Fever – – – – 9 (2.4) –
Nausea – – 4 (9.5) – 16 (4.2) –
Vomiting 1 (11.1) 1 (16.7) 4 (9.5) – 6 (1.6) 1 (2.8)
RE Induced Gastritis – – 1 (2.4) – – –
Gastritis – – – – 2 (0.5) –
RE Induced GI Ulceration – – 1 (2.4) – – –
GI Ulceration – – 1 (2.4) 1(16.7) 3 (0.8) 2 (5.6)
REILD – – 1 (2.4) 1 (16.7) – –
Radiation Pneumonitis – – – – – –
Radiation Cholecystitis – – – – – –
Radiation Pancreatitis – – – – – –
Ascites – – – – 16 (4.2) –
Dyspnoea – – 3 (7.1) 2 (33.3) 7 (1.8) 1 (2.8)
Hepatic enteropathy – – – – 8 (2.1) 4 (11.1)
Neuropathy – – – – 9 (2.4) 1 (2.8)
Hand–foot syndrome – – – – 13 (3.4) 1 (2.8)
Anorexia – – 1 (2.1) – 15 (4.0) 4 (11.1)
Constipation – – – – 9 (2.4) –
Diarrhoea – – – – 34 (8.9) 1 (2.8)
Anaemia – – – – 15 (4.0) 1 (2.8)
Jaundice – – 2 (4.8) 1 (16.7) 4 (1.0) –
Oedema – – 3 (7.2) 1 (16.7) 3 (0.8) –
Less common AEs 4 (44.4) 1 (16.7) 8 (19.1) – 72 (18.8) 8 (22.2)
Bleeding 4 (44.4) 1 (16.7) 2 (4.8) – 5 (1.3) –
Liver and portal system – – 12 (3.2) 2 (5.6)
Cardio-pulmonary – – – – 5 (1.3) –
Neurological, pain, and other
sensitive disorders
– – – – 15 (3.9) –
General – – 4 (9.5) – 9 (2.4) 1 (2.8)
Digestive – – 1 (2.4) – 9 (2.4) –
Analytical – – – – 4 (1.0) 1 (2.8)
Cutaneous complications – – 1 (2.4) – 7 (1.8) 1 (2.8)
Musculoskeletal disorders – – – – 3 (0.8) –
Pancreatitis – – – – 2 (0.5) 2 (5.6)
Renal and fluid balance – – – – 1 (0.3) 1 (2.8)
Peri–interventional \ 30 days [ 30 days
Overall Grade 3 or 4 Overall Grade 3 Overall Grade 3 or 4
Subjects with at least one AE
n = 200 (%)
8 (4) 4 (2) 24 (12) 3 (1.5) 122 (61) 28 (14)
REILD,radioembolization-induced liver disease; GI gastrointestinal
189 (94.5%) patients had no severe peri-interventional AEs. For 3 (1.5%) patients, no information was collected. Subjects with at least one peri-
interventional AEs were 8 (4%), of which 4(2%) had grade 3. There were 4 (2%) other peri-interventional AEs reported in 4 patients (three vascular
minor and one blood transfusion) no grading for these four AEs was provided. A total of 42 AEs were observed in 24 patients within 30 days
following treatment of which 6 were grade 3 (GI Ulceration, REILD, jaundice, oedema and dyspnoea). No AE grade 4 or 5 were observed during
the first 30 days after treatment
R. Loffroy et al.: Short-term Safety and Quality of Life Outcomes...
123
R. Loffroy et al.: Short-term Safety and Quality of Life Outcomes...
123
