
Improvement of dielectric properties of ZnO
nanoparticles by Cu doping for tunable microwave
devices
A. Selmi
1,
*, A. Fkiri
2
, J. Bouslimi
3,4
, and H. Besbes
5
1
Laboratory of Materials Organisation and Properties (LR99ES17), Faculty of Sciences of Tunis, University of Tunis, El Manar,
2092 Tunis, Tunisia
2
Lab of Hetero-Organic Compounds and Nanostructured Materials (LR18ES11), Faculty of Sciences of Bizerte, University of
Carthage, 7021 Zarzouna, Tunisia
3
Department of Physics, Faculty of Sciences, Taif University, Taif 888, Saudi Arabia
4
Department of Engineering Physics and Instrumentation, Institute of Applied Sciences and Technology, Carthage University, Tunis,
Tunisia
5
Department of Physics, Faculty of Science, King Abdulaziz University, Jedda, Saudi Arabia
Received: 13 May 2020
Accepted: 4 September 2020
Ó Springer Science+Business
Media, LLC, part of Springer
Nature 2020
ABSTRACT
We report a facile chemical polyol method to synthesize Cu-doped ZnO
nanoparticles with various levels of Cu. X-ray diffraction (XRD), transmission
electron microscopy (TEM), and UV–Visible diffuse reflectance spectroscopy
techniques were used to analyze the structural and optical properties of
Zn
1-x
Cu
x
O nanoparticles. The crystallite size varies between 9.8 and 18.9 nm
and decreased with the increase of Cu doping. The band energy gaps of pure
and Cu-doped ZnO samples are in the range 2.5–3.1 eV. The dielectric prop-
erties, ac conductivity and impedance analysis of Zn
1-x
Cu
x
O nanoparticles
were systematically investigated. It was revealed that the doping of ZnO by Cu
(with low Cu molar content) leads to obtain high dielectric constant and low
tangent loss, which are very encouraging for microwave semiconductor devices.
1 Introduction
In the last years, large bandgap semiconductors such
as WO
3
[1], ZnS [2], GaN [3], and ZnO [4, 5] have
been used in various applications. Among them, the
eco-friendly zinc oxide (ZnO) semiconductor mate-
rial is being one of the preferred materials for many
microelectronics applications due to their excellent
ferroelectric, photoelectric, piezoelectric, catalytic and
dielectric properties [6–11]. Furthermore, ZnO
nanoparticles have received great interest; thanks to
their potential uses in nanotechnology such as lumi-
nescence [12, 13], photo-detection [14, 15], gas sensor
[16, 17] and metal oxide semiconductor (MOS) [18].
Various methods were used to synthesize nanoma-
terials including chemical vapor deposition [19], laser
https://doi.org/10.1007/s10854-020-04408-1
J Mater Sci: Mater Electron

ablation [20], sol gel process [21], solvothermal
method [22, 23], micro-emulsion technique [24],
hydrothermal method [25], and polyol method [26].
Among them, the polyol method is a simple and low-
cost process that allows to produce ZnO nanoparti-
cles with a narrow-size distribution, a controlled
morphology, and a good crystalline quality. The
polyol solvent acts simultaneously as a complexing
agent, a surfactant, and a stabilizing agent, which
lead to avoid the agglomeration of nanoparticles [27].
The photocatalytic and magnetic properties of ZnO
are widely studied in the literature [6–18]. Never-
theless, the dielectric properties of ZnO nanoparticles
are less explored and need to be more investigated.
In recent years, researchers around the world
working on the field of semiconductor-based micro-
electronics are interesting to study the dielectric
properties of ZnO nanoparticles [28, 29]. Currently,
they seek to improve these properties, which will
make these materials good candidates for microelec-
tronic applications. To attain this objective, the con-
trol of the microstructure is important. The dielectric
properties of ZnO nanoparticles could be affected by
doping. Some types of additives or doping have been
employed such as Ni [28], Ag [30], Mn [31], Al [32],
and Co [33]. They greatly affected the dielectric
properties of ZnO nanoparticles. However, the effect
of Cu dopant on the dielectric performances of ZnO
nanoparticles has not been yet reported. Cu
2?
ion has
an ionic radius very close to the Zn
2?
one, which
indicates that Cu
2?
ions can easily enter within ZnO
crystal lattice or substitute Zn
2?
ions in the crystal
and hence can influence the properties of ZnO
properties. With this motivation, we aim in this work
to study deeply the effect of different Cu contents on
the structural, optical, and dielectric properties of
ZnO nanoparticles. Series of Zn
1-x
Cu
x
O nanoparti-
cles with various x values of 0.00, 0.05, 0.10, 0.20 and
1.00% were successfully prepared by polyol process.
2 Experimental
2.1 Synthesis of Cu-doped ZnO
nanoparticles
The preparation of undoped and Cu-doped ZnO
nanoparticles (Zn
1-x
Cu
x
O) was realized according to
a one-pot chemical reaction. Initially, copper chloride
(CuCl
2
; Sigma-Aldrich, purity 99.995%) and zinc
acetate dihydrate (Zn(OAc)
2
.2H
2
O; Sigma-Aldrich,
purity C 98%) were simultaneously mixed in 50 ml
of ethylene–glycol (EG) solvent. The contents of Cu
element are equal to 0, 0.05, 0.1, 0.2 and 1%. The
mixture was maintained under reflux for 60 min at
160 °C. The sequential reactions were thermally
controlled. Then, the attained precipitate was cen-
trifuged at 8000 rpm, washed several times (3–5
times) with ethanol, and finally dried at 100 °C for
12 h to get the final Zn
1-x
Cu
x
O powder (white color).
2.2 Characterization techniques
The structural analysis of prepared Zn
1-x
Cu
x
O
nanoparticles was characterized by X-ray diffraction
technique using a Bruker X-ray diffractometer with
Cu K
a1
radiation (k = 1.5406 A
˚
).The crystallites size
of Zn
1-x
Cu
x
O nanoparticles was calculated by
employing the Scherrer equation. The microstructure
observations were performed by transmission elec-
tron microscopy (TEM, JEOL 2100F). The optical
absorption spectra were recorded using Varian
CARY 100 Scan UV–visible diffuse reflectance spec-
trophotometer (UV–vis DRS). The dielectric proper-
ties of Zn
1-x
Cu
x
O nanoparticles were measured over
a wide frequency range (1 to 10
5
Hz) using a Solar-
tron 1260A (Impedance/Gain-Phase Analyzer) cou-
pled with 1296 Dielectric Interface.
3 Results and discussion
3.1 Characterization of Zn
12x
Cu
x
O
nanoparticles
The crystalline phase of the prepared Zn
1-x
Cu
x
O
nanoparticles was studied by XRD. Figure 1a illus-
trates the XRD patterns of Cu-doped ZnO (Zn
1-x-
Cu
x
O; where x = 0, 0.05, 0.1, 0.2 and 1). All the
diffraction peaks are indexed by the hexagonal
Wurtzite ZnO with the intense (100), (002) and (101)
characteristic peaks (space group P63mc, JCPDS No.
36-1451). The large peaks in XRD patterns indicate
that very small nanocrystals are present in the sam-
ple. The lattice parameters of the five elaborated
samples were determined by Rietveld process using
the FULLPROF program [34].The different structural
parameters of various synthesized Zn
1-x
Cu
x
O
nanoparticles including, lattice parameters, cell vol-
ume, and BET-specific surface area as a function of
J Mater Sci: Mater Electron
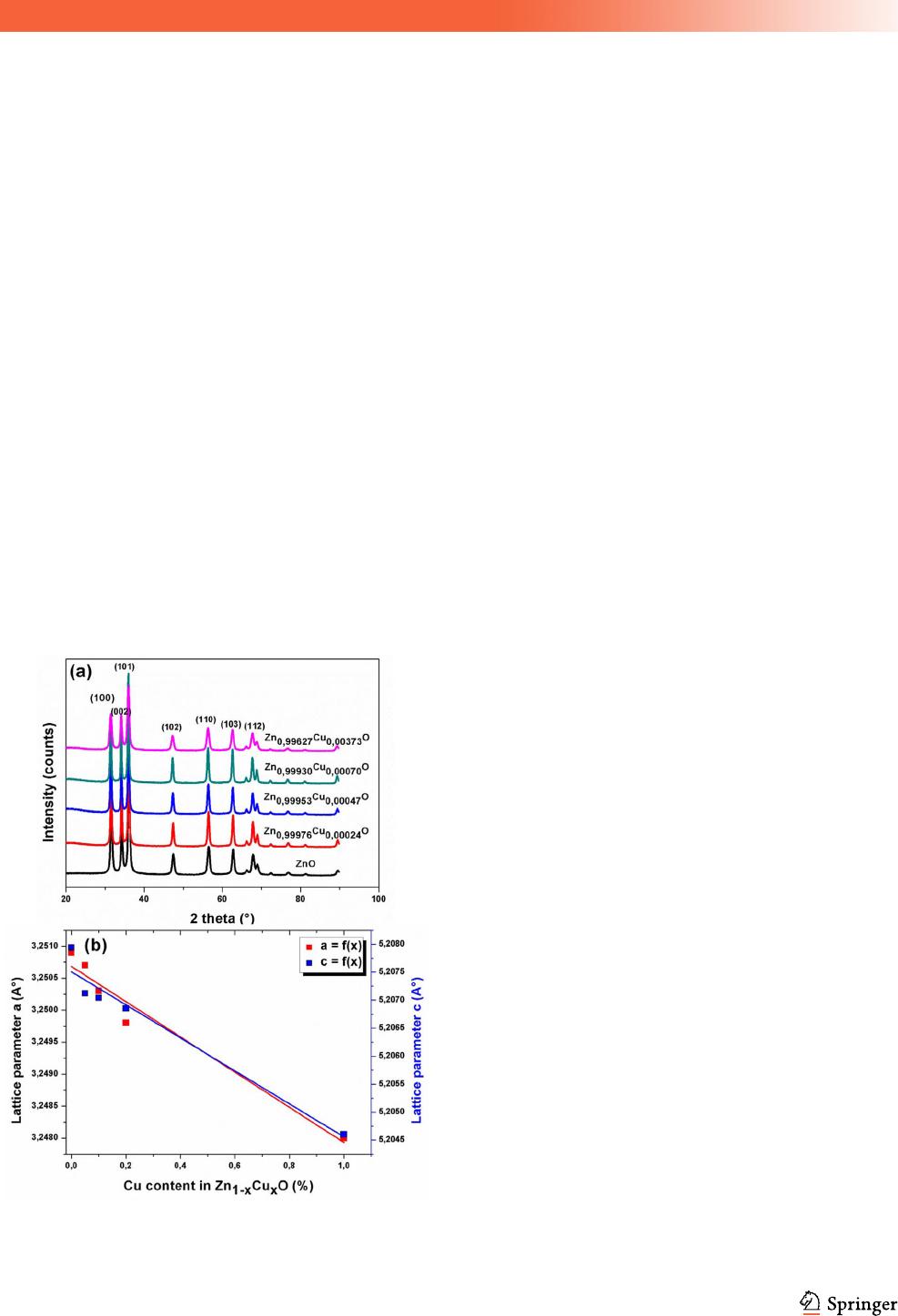
Cu concentration are summarized in Table1. The
deduced lattice parameters (a and c) decrease with
increasing the concentration of Cu dopant [35]. A
very small variation in the lattice parameters ‘a’ and
‘c’ is observed (Fig. 1b). This is due to the very close
values ionic radii of Cu
2?
(0.57 A
˚
) and Zn
2?
(0.60 A
˚
)
ions [36].
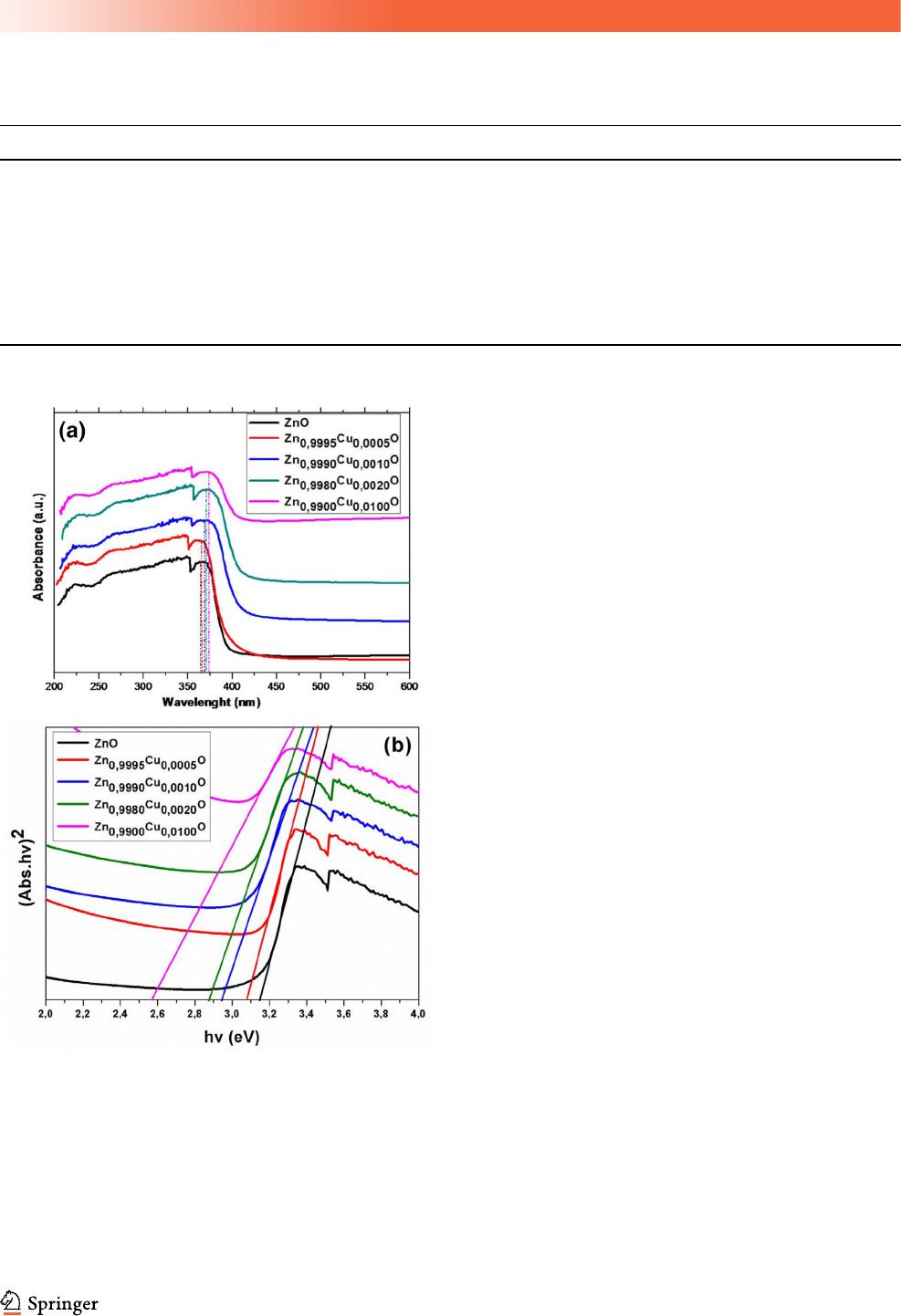
Figure 2a shows the UV–vis DRS spectra of
Zn
1-x
Cu
x
O nanoparticles. We note that the strong
absorption edge occurs in the UV light area, which is
a typical optical absorption behavior of Cu-doped
ZnO semiconductor compounds. It can be seen from
Fig. 2a that the maximum of the absorbance band
shifts slightly toward higher wavelength owing to Cu
doping. This could be mainly due to the strong
interaction between the surface oxides of Cu and Zn
[37]. The optical bandgap can be calculated from the
Tauc plot (variation of (Abs.E)
2
as a function of
photon energy E, where Abs is the absorbance) [38].
As shown in Fig. 2b, the optical bandgap obtained
from the intersection of the sharply decreasing lines
with the energy axis is 3.16, 3.08, 2.94, 2.88, and
2.57 eV for x = 0, 0.05, 0.1, 0.2 and 1, respectively
(Table 1). The obtained values confirm the quantum
confinement regime of the Cu–doped nanoparticles.
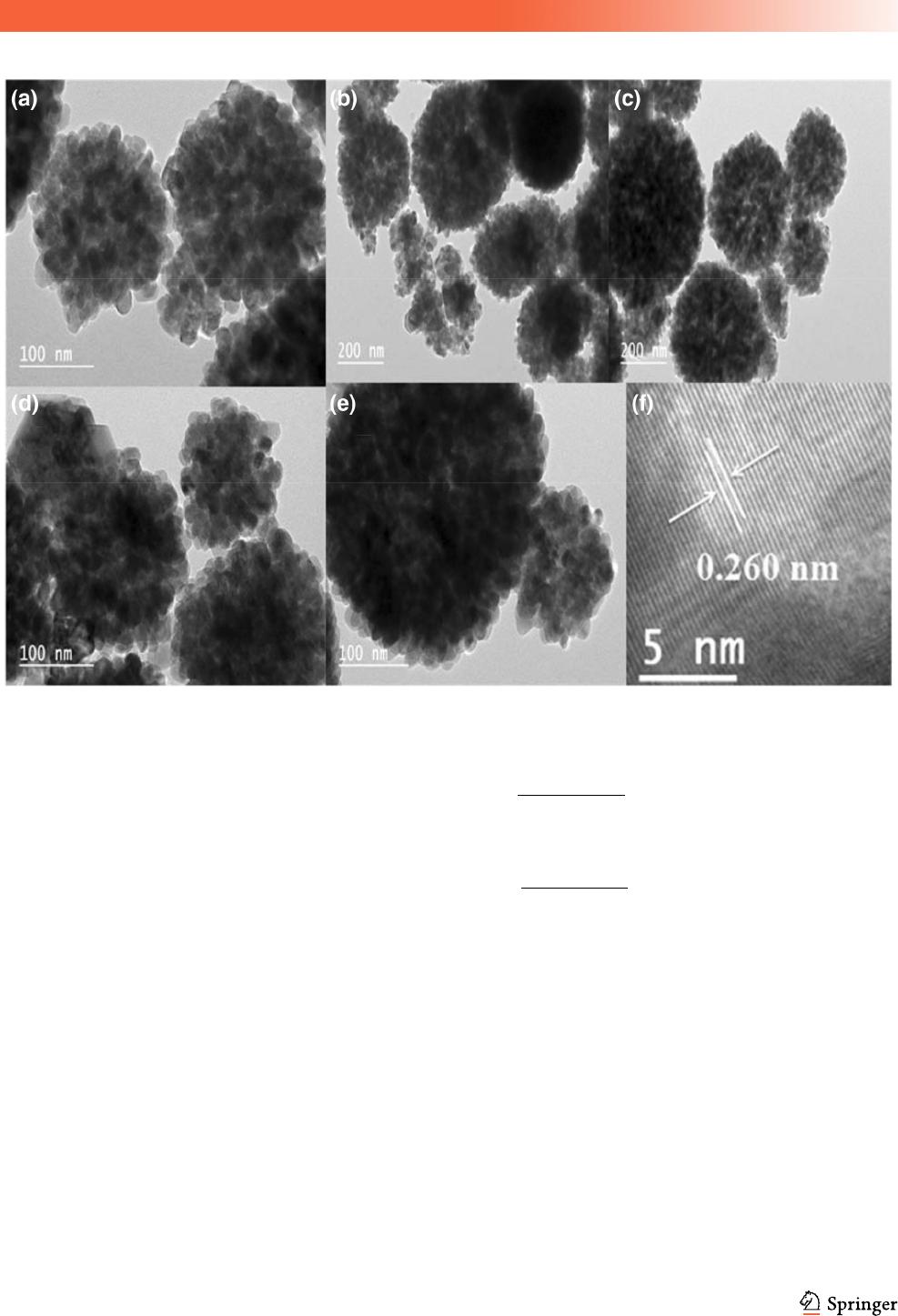
Figure 3a–f depict the TEM images obtained for
various Zn
1-x
Cu
x
O nanoparticles. TEM images
showed spherical-shaped nanoparticles with size
ranging between 9 and 20 nm (Fig. 3a–e). Figure 3f
shows a high-resolution TEM (HRTEM) micrograph
of the Zn
1-x
Cu
x
O nanoparticles with x = 0.1%. The
analysis indicates the well-defined ZnO crystal
planes, thus approving the crystalline structure of the
formed nanoparticles. The interplanar spacing of the
ZnO (002) atomic plane is 0.26 nm, which is consis-
tent with the wurtzite structure [39].
To further verify the successful preparation of
desired nanoparticles, EDAX analyses were carried
out as shown in Fig. 4. The analyses indicate that the
Zn
1-x
Cu
x
O nanoparticles are of high purity since
only Cu, Zn and O elements were detected. The
existence of Ti (at 4–5 eV) is due to titanium grid
utilized for the TEM/EDAX examination.
3.2 Dielectric and impedance studies
of Zn
12x
Cu
x
O nanoparticles
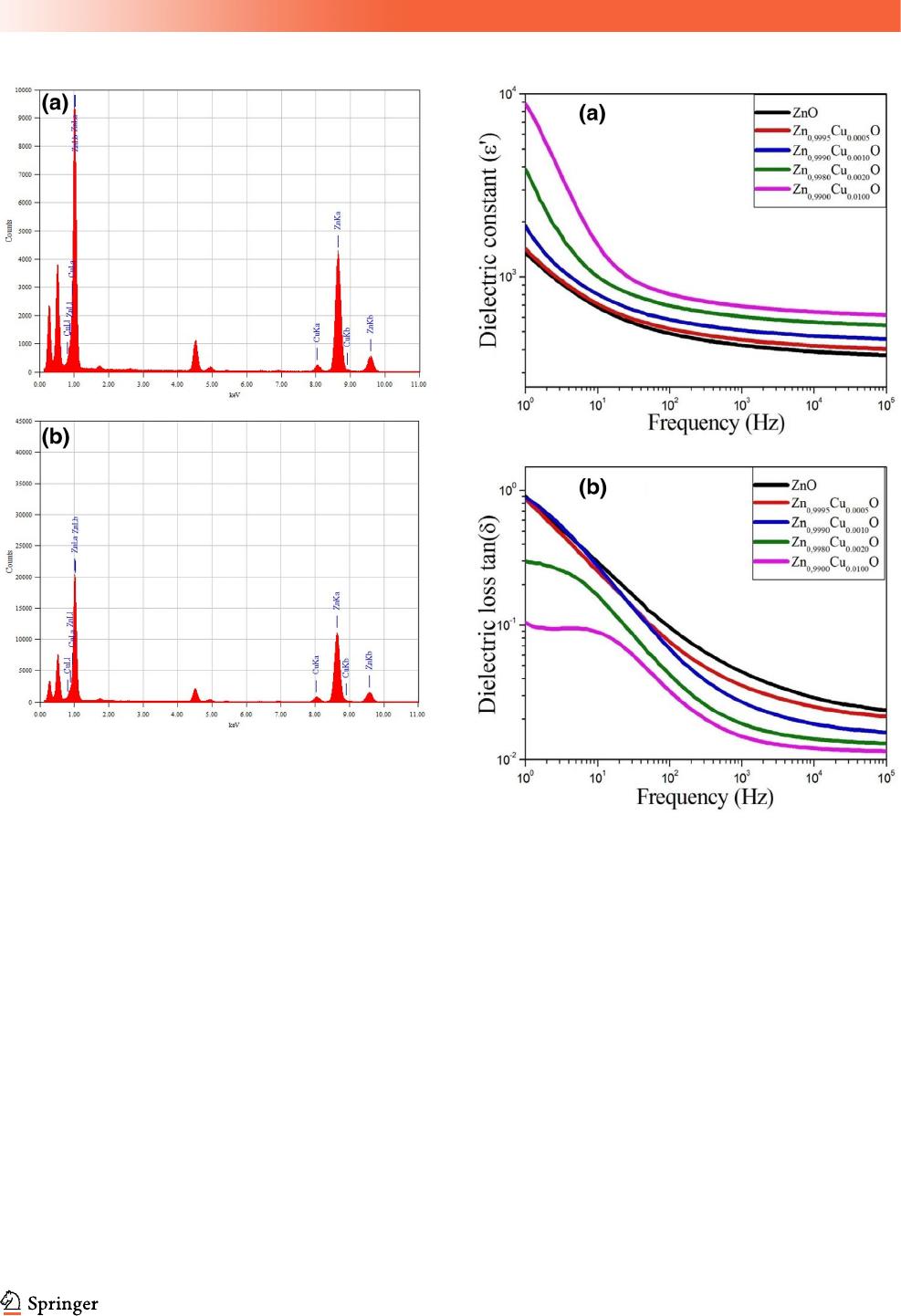
3.2.1 Dielectric properties
The study of the dielectric properties of materials is
an essential parameter for integrating these materials
into microelectronic application devices. The dielec-
tric properties of theZn
1-x
Cu
x
O (x = 0.0, 0.05, 0.1, 0.2
and 1%) nanoparticles were measured as a function
of frequency at room temperature. The variation of
dielectric constant (e
0
) for various samples is shown
in Fig. 5a. For all compositions, it is found that the
dielectric constant decreases rapidly in the low fre-
quency region, and then became more and less con-
stant at higher frequencies representing the dielectric
dispersion. The observed decrease in e
0
at the low
frequency region is a common and known feature
behavior for amorphous, polycrystalline and oxides
materials [40, 41]. This dispersion can be attributed to
the interfacial polarization (film/electrode and/or in
grain boundary interfaces) in agreement with Koop’s
phenomenological theory [42]. Starting from 100 Hz,
the decrease of the dielectric constant with the fre-
quency becomes weak and shows good stability at
high frequency. It is clear that the dielectric constant
is depended on the Cu concentration throughout the
frequency range. It increases significantly with
increasing Cu content. For example, the obtained
Fig. 1 a X-ray diffraction patterns of Zn
1-x
Cu
x
O nanoparticles,
b variation of lattice parameters as a function of Cu content
J Mater Sci: Mater Electron
permittivities at 1 kHz are equal to 422, 452, 510, 606
and 693 for x = 0.0, 0.05, 0.1, 0.2 and 1%, respectively.
It can be concluded that the increase in the Cu con-
tent within ZnO nanoparticles (even with low Cu
quantity) increases the permittivity and improves its
stability at high frequencies. The observed results are
very encouraging for microwave applications.
The frequency dependence of the dielectric loss
tangent (tand = e
00
/e
0
) for all samples at room tem-
peratures is shown in Fig. 6b. The curves of tand vs.
frequency for Zn
1-x
Cu
x
O (x = 0.0, 0.05, and 0.1%)
show the same behavior. In fact, tand decreases
rapidly with increasing frequency, showing a dis-
persion phenomenon at low frequencies. For
x = 0.2% and x = 1%, a relaxation peak was appeared
at low frequencies. The dielectric loss (tand) is also
influenced by the concentration of Cu dopant. At
1 kHz, tand values obtained are around 0.045, 0.035,
0.026, 0.018 and 0.0014 for x = 0.0, 0.05, 0.1, 0.2 and
1%, respectively. It is important to note that the
increase in Cu content within ZnO nanoparticles
leads to increase the permittivity and decrease the
dielectric loss. This implies that Cu-doped
ZnO nanoparticles are good candidates for various
microelectronic applications such as sensors, memory
devices, actuators, MOS capacitors and semiconduc-
tor microwave devices.
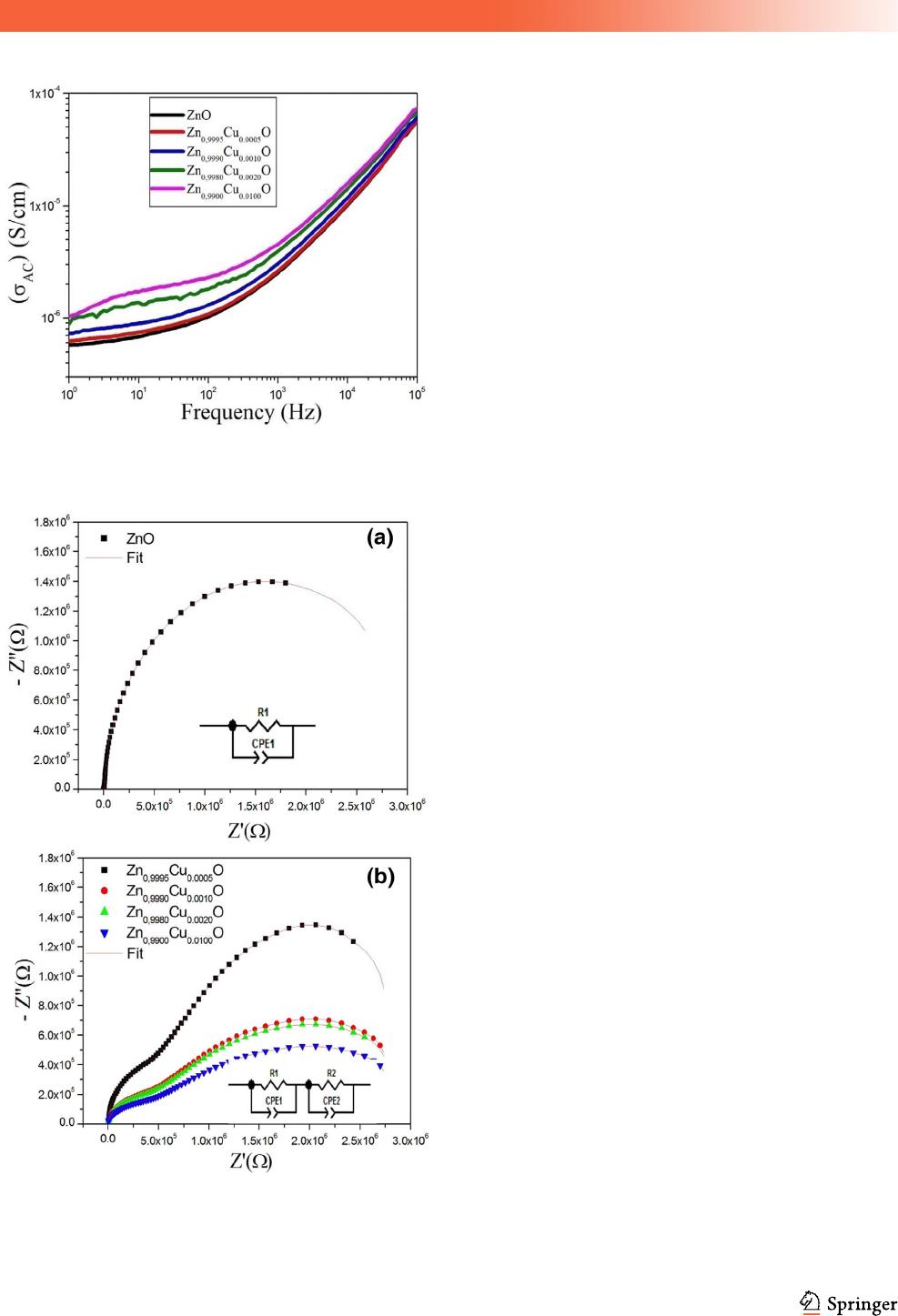
3.2.2 AC conductivity investigation
Using the data of e
0
and tan(d), the ac electrical con-
ductivity (r
ac
) of the Zn
1-x
Cu
x
O nanoparticles can be
calculated from the empirical formula: r
ac
= xe
0
e
0
tan(d), where x is the angular frequency and e
0
is the
vacuum permittivity [43]. Figure 6 shows the fre-
quency dependence of ac conductivity (r
ac
) at room
temperature for pure and Cu-doped ZnO nanoparti-
cles. One can observe two regions; the conductivity is
almost frequency independent in the low frequency
region, while it is strongly dependent on frequency at
the high frequency region (starting from 100 Hz).
Table 1 Molar Cu concentration, lattice parameters, cell volume, BET-specific surface area, crystallites size and bandgap for Zn
1-x
Cu
x
O
nanoparticles
Cu content in the synthesis mixture 0.00% 0.05% 0.10% 0.20% 1.00%
Cu content in Zn
1-x
Cu
x
O 0.00% 0.02% 0.05% 0.07% 0.37%
a(A
˚
) 3.2509 (3) 3.2502 (3) 3.2494 (2) 3.2485 (8) 3.2480 (2)
c(A
˚
) 5.2079 (4) 5.2071 (2) 5.2070 (4) 5.2068 (5) 5.2045 (2)
Cell volume (A
˚
3
) 48.5876 (2) 48.5562(1) 48.5248 (3) 48.4801 (1) 48.4577 (2)
BET surface area (m
2
/g) 21 ± 320± 220± 219± 318± 2
Crystallites size D
XRD
(± 1 nm)
18.9 17.5 15.3 12.7 9.8
Bandgap (eV) 3.16 3.08 2.94 2.88 2.57
Fig. 2 a UV–visible diffuse reflectance spectra, and b Tauc plots
for pure and Cu-doped ZnO nanoparticles
J Mater Sci: Mater Electron
Therefore, the ac conductivity can be described by the
Jonscher’s power law: r
ac
= r
dc
? A x
n
where r
dc
is
the dc bulk conductivity, A is a pre-exponential
constant, and n is the power law exponent [44]. One
can observe that the conductivity of the Zn
1-x
Cu
x
O
nanoparticles increases with the increase in Cu con-
tent. This increase is clearer in the low frequencies
region.
3.2.3 Complex impedance analysis
For polycrystalline structure, it is always better to
separate the effects of the dissociation between the
bulk and the interface. Accordingly, we investigated
the complex impedance spectra of Zn
1-x
Cu
x
O
nanoparticles. The complex impedance (Z
*
) can be
written in the following form: Z
*
= Z
0
- jZ
00
. The real
and imaginary parts (Z
0
and Z
00
) of the complex
impedance can be also expressed as a function of e
0
and e
00
as shown in the following expressions:
Z
0
¼
e
00
xC
0
e
02
þ e
002
ðÞ
and
Z
00
¼
e
0
xC
0
e
02
þ e
002
ðÞ
where e
0
and e
00
are the real and imaginary parts of the
complex permittivity (e
*
), x =2pf is the angular fre-
quency, C
0
= e
0
A/t is the geometrical capacitance, e
0
is the permittivity of free space, A is the area of
electrode surface and t is the thickness. Figure 7a
shows the Nyquist plot (Z
00
versus Z
0
) at room tem-
perature for Zn
1-x
Cu
x
O nanoparticles (x = 0.0%, 0.05,
0.1, 0.2 and 1%). The pure ZnO nanoparticles showed
only one semi-circle, while the Cu-doped ZnO
nanoparticles showed two semi-circles. The semi-
circle observed at the lower frequency region is
attributed to the contribution of grain boundary and
the interfacial effect specified by the interface
between the Cu and the ZnO nanoparticles
Fig. 3 TEM micrographs of Zn
1-x
Cu
x
O; a x = 0.00%, b x = 0.05%, c x = 0.1%, d x = 0.2% and e x = 1%, and f HRTEM micrograph
of ZnO nanoparticles
J Mater Sci: Mater Electron
(interfacial properties), while the semi-circle occurred
at the higher frequency region is associated to the
grain effect (bulk properties). By using Z-View 2
software, the experimental impedance measurements
were best fitted with the equivalent circuit based on
the brick-layer model as shown in Fig. 7a and b. For
pure ZnO nanoparticles, the equivalent circuit is
composed of a one-cell constituted by a parallel
combination of Rp and CPE. The equivalent circuit
obtained for the doped Zn
1-x
Cu
x
O nanoparticles is
formed by two cells connected in series each con-
sisting of a parallel combination of Rp and CPE
representing grain and grain boundaries effects,
respectively.
4 Conclusion
In this study, we have prepared Zn
1-x
Cu
x
O
nanoparticles (where x = 0.00, 0.05, 0.10, 0.20 and
1.00%) using a one-pot polyol process, without add-
ing any other reagent, template or complex metal
ligand. XRD analysis showed the successful forma-
tion of the Zn
1-x
Cu
x
O nanoparticles with hexagonal
wurtzite structure. TEM and EDAX analyses showed
high purity of Cu-doped ZnO nanoparticles with
spherical shape and a size ranging between 9 and
20 nm. HRTEM image showed well-defined ZnO
crystal planes. The investigation of electrical and
dielectric properties indicated that the increase in the
Fig. 4 EDAX spectra of Zn
1-x
Cu
x
O nanoparticles; a x = 0.05%
and b x = 0.1%
Fig. 5 Frequency dependence of dielectric constant (e
0
) and
tangent loss (tan d) forZn
1-x
Cu
x
O nanoparticles measured at room
temperature (25 °C)
J Mater Sci: Mater Electron
Cu content improves greatly the dielectric properties
of Zn
1-x
Cu
x
O nanoparticles. It is found that the
permittivity increased and the dielectric loss
decreased with Cu doping. The obtained results are
very encouraging to use the Zn
1-x
Cu
x
O nanoparticles
in semiconductor microwave devices.
Acknowledgements
This project was funded by the Deanship of Scientific
Research (DSR), King Abdulaziz University, Jeddah,
under grant. No. (D-625-130-1441). The authors,
therefore, gratefully acknowledge DSR technical and
financial supports.
References
1. L. Santos, C.M. Silveira, E. Elangovan, J.P. Neto, D. Nunes,
L. Pereira, R. Martins, J. Viegas, J.J. Moura, S. Todorovic,
M.G. Almeida, Synthesis of WO3 nanoparticles for biosens-
ing applications. Sens. Actuators B 223, 186–194 (2016)
2. X. Wang, H. Huang, B. Liang, Z. Liu, D. Chen, G. Shen, ZnS
nanostructures: synthesis, properties, and applications. Crit.
Rev. Solid State Mater. Sci. 38(1), 57–90 (2013)
3. Y. Al-Douri, Optical properties of GaN nanostructures for
optoelectronic applications. Procedia Eng. 53, 400–404
(2013)
4. P.K. Mishra, H. Mishra, A. Ekielski, S. Talegaonkar, B.
Vaidya, Zinc oxide nanoparticles: a promising nanomaterial
for biomedical applications. Drug Discov. Today 22,
1825–1834 (2017)
5. E. Manikandan, V. Murugan, G. Kavitha, P. Babu, M. Maaza,
Nanoflower rod wire-like structures of dual metal (Al and Cr)
doped ZnO thin films: structural, optical and electronic
properties. Mater. Lett. 131, 225–228 (2014)
6. R. Tayebee, A.H. Nasr, S. Rabiee, E. Adibi, Zinc oxide as a
useful and recyclable catalyst for the one-pot synthesis of
2,4,6-trisubstituted-1,3,5-trioxanes under solvent-free condi-
tions. Eng. Chem. Res. 52, 9538–9543 (2013)
7. A.H. Shah, M.B. Ahamed, E. Manikandan, R. Chandramo-
han, M. Iydroose, Magnetic, optical and structural studies on
Ag doped ZnO nanoparticles. J. Mater. Sci.: Mater. Electron.
24, 2302–2308 (2013)
8. M. Kaushik, R. Niranjan, R. Thangam, B. Madhan, V.
Pandiyarasan, C. Ramachandran, D.H. Oh, G.D. Venkata-
subbu, Investigations on the antimicrobial activity and wound
healing potential of ZnO nanoparticles. Appl. Surf. Sci. 479,
1169–1177 (2019)
Fig. 6 Variation of ac conductivity (r
ac
) with frequency for
Zn
1-x
Cu
x
O nanoparticles
Fig. 7 Typical Nyquist Plot for Zn
1-x
Cu
x
O nanoparticles at room
temperature and the corresponding equivalent circuit
J Mater Sci: Mater Electron

9. K. Lokesh, G. Kavitha, E. Manikandan, G.K. Mani, K.
Kaviyarasu, J.B. Rayappan, R. Ladchumananandasivam, J.S.
Aanand, M. Jayachandran, M. Maaza, Effective ammonia
detection using n-ZnO/p-NiO heterostructured nanofibers.
IEEE Sens. J. 16(8), 2477–2483 (2016)
10. S. Goel, B. Kumar, A review on piezo-/ferro-electric prop-
erties of morphologically diverse ZnO nanostructures. J. Al-
loys Compds. 816, 152491 (2020)
11. H. Agarwal, S.V. Kumar, S. Rajeshkumar, A review on green
synthesis of zinc oxide nanoparticles—an eco-friendly
approach. Resource Effic. Technol. 3(4), 406–413 (2017)
12. R. Raji, K.G. Gopchandran, ZnO nanostructures with tunable
visible luminescence: effects of kinetics of chemical reduction
and annealing. J. Sci. Adv. Mater. Dev. 2, 51–58 (2017)
13. B.D. Ngom, T. Mpahane, E. Manikandan, M. Maaz, ZnO
nano-discs by lyophilization process: size effects on their
intrinsic luminescence. J. Alloy Compd. 656, 758–763 (2016)
14. S.P. Chang, K.J. Chen, Zinc oxide nanoparticle photodetector.
J. Nanomater. 602398 (2012)
15. A. Muthukumar, D. Arivuoli, E. Manikandan, M. Jayachan-
dran, Enhanced violet photoemission of nanocrystalline flu-
orine doped zinc oxide (FZO) thin films. Opt. Mater. 47,
88–94 (2015)
16. S. Bhatia, N. Verma, R.K. Bedi, Ethanol gas sensor based
upon ZnO nanoparticles prepared by different techniques.
Results Phys. 7, 801–806 (2017)
17. G. Kavitha, K. Thanigai Arul, P. Babu, Enhanced acetone gas
sensing behavior of n-ZnO/p-NiO nanostructures. J. Mater.
Sci.: Mater. Electron. 2(8), 6666–6671 (2018)
18. L. Chen, Z. Yu-Ming, Z. Yi-Men, L. Hong-Liang, Interfacial
characteristics of Al/Al
2
O
3
/ZnO/n-GaAs MOS capacitor.
Chin. Phys. B 22(7), 076701 (2013)
19. M. Laurenti, N. Garinoa, S. Porro, M. Fontana, C. Gerbaldi,
Zinc oxide nanostructures by chemical vapour deposition as
anodes for Li-ion batteries. J. Alloy Compd. 640, 321–326
(2015)
20. K.K. Kim, D. Kim, S.K. Kim, S.M. Park, J.K. Song, For-
mation of ZnO nanoparticles by laser ablation in neat water.
Chem. Phys. Lett. 511(1–3), 116–120 (2011)
21. N.T. Rochman, A.P. Riski, Fabrication and characterization of
Zinc Oxide (ZnO) nanoparticle by sol-gel method. JPhCS.
853(1), 012041 (2017)
22. P. Veluswamy, S. Suhasini, F. Khan, A. Ghosh, M. Abhijit, Y.
Hayakawa, H. Ikeda, Incorporation of ZnO and their com-
posite nanostructured material into a cotton fabric platform
for wearable device applications. Carbohydr. Polym. 157,
1801–1808 (2016)
23. P. Veluswamy, S. Suhasini, J. Archana, M. Navaneethan, A.
Majumdar, Y. Hayakawa, H. Ikeda, Fabrication of
hierarchical ZnO nanostructures on cotton fabric for wearable
device applications. Appl. Surf. Sci. 418, 352–361 (2017)
24. O
¨
.A. Yıldı r ım, C. Durucan, Synthesis of zinc oxide
nanoparticles elaborated by micro-emulsion method. J. Alloy.
Compd. 506 , 944–949 (2010)
25. D.B. Bharti, A.V. Bharati, Synthesis of ZnO nanoparticles
using a hydrothermal method and a study its optical activity.
Luminescence 32, 317–320 (2017)
26. B.W. Chieng, Y.Y. Loo, Synthesis of ZnO nanoparticles by
modified polyol method. Mater. Lett. 73, 78–82 (2012)
27. F. Fie´vet, S. Ammar-Merah, R. Brayner, F. Chau, M. Giraud,
F. Mammeri, J. Peron, J.-Y. Piquemal, L. Sicard, G. Viau, The
polyol process: a unique method for easy access to metal
nanoparticles with tailored sizes, shapes and compositions.
Chem. Soc. Rev. 47(47), 5187–5233 (2018)
28. S. Sharma, K. Nanda, R.S. Kundu, R. Punia, N. Kishore,
Structural properties, conductivity, dielectric studies and
modulus formulation of Ni modified ZnO nanoparticles.
J. Atom. Mol. Condens. Nano Phys. 2, 15–31 (2015)
29. C. Thenmozhi, V. Manivannan, E. Kumar, S. Veera, R.
Murugan, Structural and frequency dependent dielectric
properties of ZnO nanoparticles and PANI/ ZnO nanocom-
posites by microwave—assisted solution method. Int. J. Adv.
Res. 4, 572–578 (2016)
30. I. Ahmad, M.E. Mazhar, M.N. Usmani, K. Khan, S. Ahmad,
J. Ahmad, Impact of silver dopant on electrical and dielectric
properties of ZnO nanoparticles. Mater. Res. Express 6,
035014 (2019)
31. C. Belkhaoui, R. Lefi, N. Mzabi, H. Smaoui, Synthesis,
optical and electrical properties of Mn doped ZnO nanopar-
ticles. J. Mater. Sci.: Mater. Electron. 29, 7020–7031 (2018)
32. R. Zamiri, B. Singh, M.S. Belsley, J.M. Ferreira, Structural
and dielectric properties of Al-doped ZnO nanostructures.
Ceram. Int. 40, 6031–6036 (2014)
33. A. Franco Jr., H.V.S. Pessoni, Enhanced dielectric constant of
Co-doped ZnO nanoparticulate powders. Phys. B 476, 12–18
(2015)
34. H.M. Rietveld, Line profiles of neutron powder-diffraction
peaks for structure refinement. J. Acta Cryst. 22, 151–152
(1967)
35. C.J. Rodriguez, A Program for Rietveld Refinement and
Pattern Matching Analysis,’’ Abstract of the Satellite Meeting
on Powder Diffraction of the XV Congress of the IUCr,
Collected Abstract of Powder Diffraction Meeting. Toulouse,
France, (1990) 127.
36. R.D. Shannon, Revised effective ionic radii and systematic
studies of interatomic distances in halides and chalcogenides.
Acta Cryst. A 32, 751–767 (1976)
J Mater Sci: Mater Electron

37. M. Fu, Y. Li, S. Wu, P. Lu, J. Liu, F. Dong, Sol-gel prepa-
ration and enhanced photocatalytic performance of Cu-doped
ZnO nanoparticles. Appl. Surf. Sci. 258, 1587–1591 (2011)
38. M. Moffitt, A. Eisenberg, Size control of nanoparticles in
semiconductor-polymer composites. 1. Control via multiplet
aggregation numbers in styrene-based random ionomers.
J. Chem. Mater. 7, 1178–1184 (1995)
39. C. Wu, L. Shen, H. Yu, Y.C. Zhang, Q. Huang, Solvothermal
synthesis of Cu-doped ZnO nanowires with visible light-dri-
ven photocatalytic activity. J. Mater Lett. 74, 236–238 (2012)
40. A. Selmi, M. Mascot, F. Jomni, J.-C. Carru, Investigation of
interfacial dead layers parameters in Au/Ba0.85Sr0.15TiO3/Pt
capacitor devices. J. Alloys Compds. 826, 154048 (2020)
41. K. Jeyasubramaniana, R.V. William, P. Thiruramanathan,
G.S. Hikku, M. Vimal Kumar, B. Ashima, P. Veluswamy, H.
Ikeda, Dielectric and magnetic properties of nanoporous
nickel doped zinc oxide for spintronic applications. J. Magn.
Magn. Mater. 485, 27–35 (2019)
42. Y. Slimani, A. Selmi, E. Hannachi, M.A. Almessiere, A.
Baykal, I. Ercan, Impact of ZnO addition on structural,
morphological, optical, dielectric and electrical performances
of BaTiO3 ceramics. J. Mater. Sci.: Mater. Electron. 30,
9520–9530 (2019)
43. A. Selmi, M. Mascot, F. Jomni, J.-C. Carru, High tunability in
lead-free Ba0.85Sr0.15TiO3thick films for microwave tun-
able applications. Ceram. Int. B 45, 22445–23856 (2019)
44. A. Selmi, O. Khaldi, M. Mascot, F. Jomni, J.-C. Carru,
Dielectric relaxations in Ba
0.85
Sr
0.15
TiO
3
thin films deposited
on Pt/Ti/SiO
2
/Si substrates by sol–gel method. J. Mater. Sci.:
Mater. Electron. 27, 11299–11307 (2016)
Publisher’s Note Springer Nature remains neutral with
regard to jurisdictional claims in published maps and
institutional affiliations.
J Mater Sci: Mater Electron
