
Vol.:(0123456789)
1 3
Breast Cancer
https://doi.org/10.1007/s12282-020-01129-5
CASE REPORT
Kounis syndrome afterpatent blue dye injection forsentinel lymph
node biopsy
MaximosFrountzas
1
· PanagiotisKarathanasis
1
· GavriellaZoiVrakopoulou
1
· CharalamposTheodoropoulos
1
·
ConstantinosG.Zografos
2
· DimitriosSchizas
2
· GeorgeC.Zografos
1
· NikolaosV.Michalopoulos
1,3
Received: 11 May 2020 / Accepted: 22 June 2020
© The Japanese Breast Cancer Society 2020
Abstract
Background Kounis syndrome (KS) has been described as an acute coronary syndrome (ACS) associated with an anaphy-
lactic reaction. Several triggers have been identified and the diagnostic and treatment process can be challenging.
Case A 58-year-old, female patient diagnosed with breast cancer and no history of allergies had subcutaneous injection
of patent blue V dye for sentinel lymph node biopsy (SLNB). Intraoperatively, she developed anaphylactic shock and was
transferred to the intensive care unit (ICU). A few hours later, electrocardiographic alterations and elevation of blood troponin
were observed. Emergency coronary angiography revealed no occlusive lesions in coronary vessels. Further investigation in
the allergy department set the diagnosis of KS.
Conclusion There are just ten cases of perioperative KS in the literature so far and here we present the first one triggered by
patent blue V dye for sentinel node biopsy.
Keywords Kounis syndrome· Patent blue· Sentinel node biopsy
Introduction
Sentinel lymph node biopsy (SLNB) has been considered as
the gold standard method for axillary staging in early breast
cancer. Surgeons traditionally utilize intra-parenchymal
injection of blue dye, either alone or in combination with
a radiotracer [1]. Patent blue violet (V) has been the most
commonly used blue dye outside the USA, despite its minor
allergic reaction risk, which is below 1% [2]. Patent blue V
allergic reactions could vary from simple cutaneous mani-
festations to cardiac arrest [3].
Kounis Syndrome (KS) is an extremely rare anaphylactic
reaction to various allergens, which is associated with acute
coronary syndrome [4]. KS leads to hypovolemic shock of
cardiogenic etiology, that actually should be treated as an
anaphylactic one. Herein we present the first case in the
literature concerning KS after patent blue V injection for
SLNB in early-stage breast cancer.
Case report
A 58-year-old Caucasian woman with an invasive ductal car-
cinoma in the upper outer quadrant of the right breast was
scheduled for breast conserving operation and axillary sen-
tinel lymph node biopsy. Her past medical history included
only a mild dilatation of the thoracic aorta (4.2cm). No
allergies were reported. The preoperative echocardiogram
revealed no abnormalities with a left ventricular ejection
fraction of 55–60%. During the operation, 2ml of patent
blue V was administered subcutaneously in the periareolar
area of the right breast.
Approximately 15min after patent blue V dye injection,
the patient progressively developed tachycardia (110bpm)
followed by hypotension (blood pressure: 60/30mmHg).
* Maximos Frountzas
1
1st Department ofPropaedeutic Surgery, Medical School,
“Hippocratio” General Hospital, University ofAthens, 114
Vas. Sophias Av, 11527Athens, Greece
2
1st Department ofSurgery, Medical School, University
ofAthens, “Laiko” General Hospital, 17 Agiou Thoma St,
11527Athens, Greece
3
4th Dept. ofSurgery, Medical School, “Attikon” University
Hospital, University ofAthens, 1 Rimini St, 12462Chaidari,
Greece

Breast Cancer
1 3
Pulse oximetry decreased from 100 to 95%. The patient
developed a rash initially in the face, which gradually
expanded in the whole body, and a systemic allergic reac-
tion was diagnosed. The patient immediately received
bolus doses of corticosteroids and dimetindene. In addi-
tion, fluid resuscitation with 3L of crystalloids and doses
of norepinephrine were administered to maintain blood
pressure in normal values. High-sensitivity (HS) troponin-
I levels were normal (2.23mg/dl).
The patient was transferred to the intensive care unit
(ICU) for monitoring. 6h later, electrocardiogram abnor-
malities (depression of ST segment in leads II and III)
were observed (Fig.1). New blood samples for HS tro-
ponin-I were abnormal (2360mg/dl). She underwent a
new echocardiogram, which demonstrated a decrease of
ejection fraction (40%) and a hypokinetic left cardiac ven-
tricle. The patient was treated with dual antiplatelet ther-
apy (clopidogrel 300mg and acetylsalicylic acid 100mg)
and underwent emergent coronary angiography, which
revealed no occlusive lesions in the coronary arteries, but
confirmed the low ejection fraction (40%). The follow-
ing 24h her condition gradually improved and she was
extubated the next day, being haemodynamically stable
and without need of vasopressor support. After Intensive
Care Unit discharge, the levels of HS troponin-I gradually
decreased until they became normal, and she was finally
discharged from hospital on the sixth postoperative day.
A month later, the patient was reviewed at the Allergy
and Immunology Department, where allergy to patent blue
V was confirmed.
Discussion
Kounis syndrome (KS), or “allergic angina syndrome”,
is defined as the co-incidental occurrence of an acute
coronary syndrome (ACS) associated with mast cell and
platelet activation in the setting of an allergic or anaphy-
lactic insult [5]. Its annual incidence is 4 to 10 cases per
100,000 inhabitants and its mortality rate is 0.0001% [6,
7]. Although KS could be presented in any age group,
68% of patients affected are between 40 and 70years. Risk
factors of KS include history of allergy, hypertension,
smoking, diabetes and hyperlipidemia. The most common
triggers of KS are antibiotics (27.4%) followed by insect
bites (23.4%), even though more triggers are still under
investigation [8].
Three main types of KS have been described so far:
•
Type I (without coronary disease, 75%): patients with-
out previous history of coronary disease, who present
anaphylactic reaction that causes coronary vessels
spasm. Electrocardiographic changes cardiac enzymes
elevation could be present. Endothelial dysfunction
and/or microvascular angina have been proposed as
probable pathophysiological mechanisms of this type
of KS [9].
•
Type II (with coronary disease, 20%): patients with pre-
existing atheromatous disease, either symptomatic or
unknown, in which the hypersensitivity reaction causes
plaque erosion or rupture, leading to acute myocardial
infarction [10].
•
Type III (with stents, 5%): patients with previous cor-
onary artery disease and drug-eluting stents that get
thrombosed due to hypersensitivity vasculitis [11].
KS is characterized by the interaction between mast cells,
macrophages and T-lymphocytes, which activates an inflam-
mation cascade that is mediated by several molecules and
leads to coronary artery spasm and/or atheromatous plaque
erosion or rupture during an allergic reaction [12, 13]. Mast
cells are located in the areas of coronary plaque erosion or
rupture, whereas they act on the smooth muscle of the coro-
nary arteries [14]. The inflammation cascade is triggered
by antigen–antibody reaction on the surface of the mast and
basophil cells, or activation of the complement system (C3a,
C5a), which explains the rapid—less than 60min—manifes-
tation of KS after trigger exposure [15]. In addition, there is
a threshold beyond which the inflammation cascade is acti-
vated, that depends on the body site, where the antigen–anti-
body reaction occurs, the area of exposure, the mediators
release and the severity of the allergic reaction [16].
Therefore, it is understandable why KS is exacerbated
very quickly with high severity perioperatively, where the
Fig. 1 Electrocardiogram showing alterations (ST segment depres-
sion in leads II and III) developed during Intensive Care Unit stay

Breast Cancer
1 3
potential triggers are administrated in the blood circula-
tion, either during general anesthesia induction or during
an operation. Only ten cases of perioperative KS have been
reported in the literature so far (Table1). In six of them,
KS was presented during general anesthesia induction or
immediately after that, but before skin incision. The rest
four cases were reported intraoperatively after skin inci-
sion by surgeons. Several triggers have been reported to
cause perioperative KS such as rocuronium, cefazoline,
gelofusine, midazolam, bupivacaine, latex and succi-
nylated gelatin. In 80% of the patients that experienced a
perioperative KS, there was no history of allergy or previ-
ous coronary disease (Type I).
Perioperative KS has presented as hypovolemic shock—
hypotension and tachycardia—during anesthesia induction
or within 60min after it. However, only in four out of ten
reported cases electrocardiographic alterations and rash were
initially manifested. In four more cases ACS was identified
by electrocardiographic alterations without clinical signs of
anaphylactic reaction. Finally, in two cases allergic reac-
tion was the first manifestation with a rash, but no initially
clinical signs of a coronary syndrome. Simultaneous clinical
signs of ACS and allergic reaction impose a serious diagnos-
tic problem and one out of ten patients died due to periop-
erative KS. All cases were managed according to ACS and
anaphylactic reaction therapeutic protocols.
KS in the operating room may prove serious and usually
presents as a hypovolemic shock. The key for successful
management of such a shock in an intubated patient is recog-
nizing the cause. This may prove to be challenging, as only
40% of patients develop a combination of ACS and allergic
clinical signs. Similar to what happened in our case, 20% of
patients with perioperative KS initially manifest an anaphy-
lactic reaction without ACS. Therefore, they are generally
treated for anaphylactic shock, as the pathophysiologic basis
of coronary occlusion in KS is based on hypersensitivity
reactions, that are resolved by corticosteroids and antihis-
tamines [17]. The main problem, however, exists in 40% of
patients with perioperative KS, that present ACS without
an initial anaphylactic reaction. These patients are treated
according to an ACS therapeutic protocol that is usually
not effective, because it actually does not deal with the real
cause of coronary occlusion [18]. The key for such patients
is raised suspicion by anesthesiologists and surgeons, with
continuous assessment of their whole body for clinical signs
of allergic reaction.
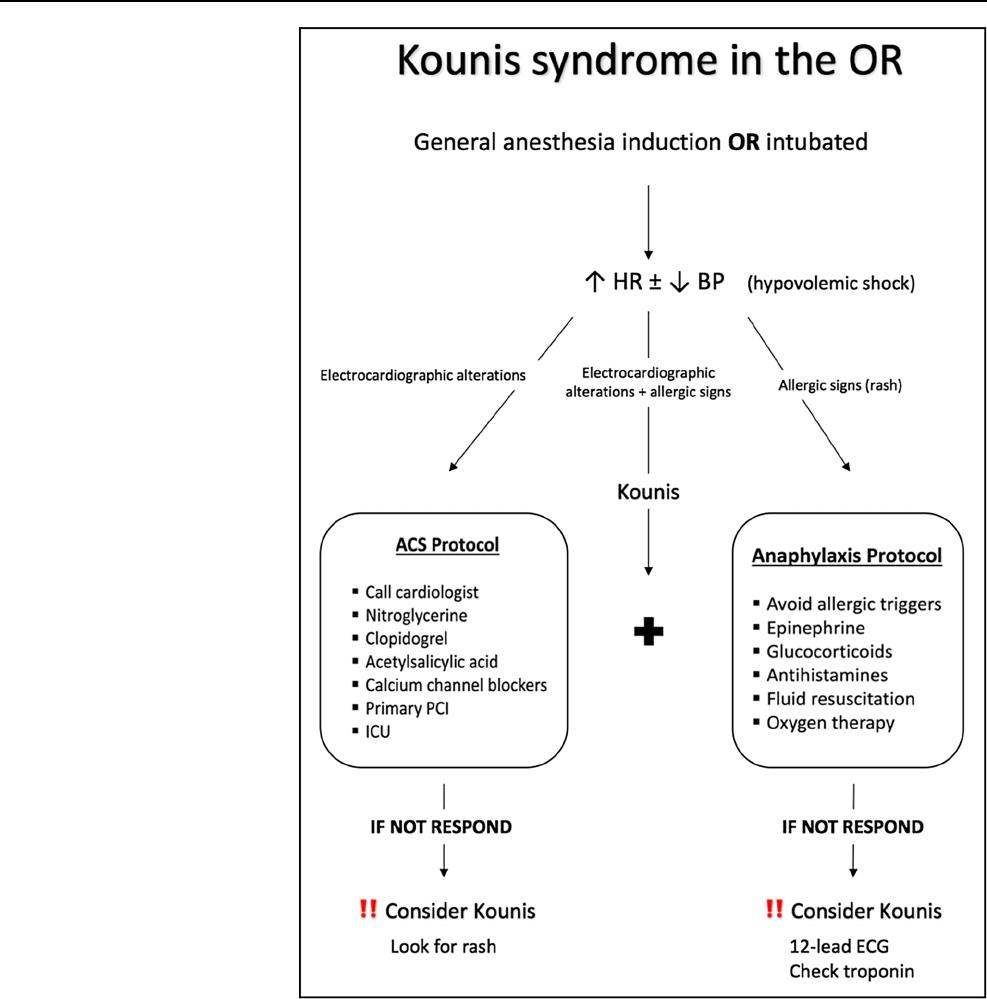
A possible algorithm for treating perioperative KS is shown
in Fig.2. When its anaphylactic component is present, all aller-
gic triggers should be removed, epinephrine, glucocorticoids
and antihistamines should be administered in combination to
high flow oxygen and fluid resuscitation [19]. When its coro-
nary syndrome component is present, a cardiology review and
administration of nitroglycerine, double antiplatelet medica-
tion (clopidogrel and acetylsalicylic acid) and calcium chan-
nel blockers (diltiazem, verapamil) is advised [20]. Primary
percutaneous coronary intervention (PCI) and ICU monitoring
are usually indicated Fig. (2).
In our case, patent blue V dye has triggered KS. Several
allergic reactions against patent blue V have been reported
in the literature, the incidence of which varies between 0.06
and 2.7%, with a mean value of 0.71%. Patent blue V allergic
reactions could vary from simple cutaneous manifestations to
cardiac arrest. Fortunately, the reported risk of severe allergic
reactions, that required vasopressors administration or surgery
interruption, remained extremely low and barely reached 0.1%
[21]. In addition, methylene blue has been proposed as a poten-
tial alternative to patent blue V, due to its comparable efficacy
in SLN identification, its lower cost and its decreased allergic
stimulation (below 0.5%). However, the possibility for cross
reactivity between these two dyes still exists [22]. Moreover,
super paramagnetic iron oxide (SPIO) nanoparticles and indo-
cyanine green fluorescence (ICG) have demonstrated adequate
efficiency in SLN mapping in combination to a radio-isotope,
but their safety has still to be proved [23, 24]. Finally, the uti-
lization of a radio-isotope alone without a blue dye is another
proposed option that showed comparable SLN identification
rates, but required advanced surgical experience [25]. Nev-
ertheless, patent blue V has never been reported as a trigger
for KS.
Conclusion
KS is a combination of ACS and anaphylactic reaction that
rarely happens in the operating room. Diagnosis demands
raised suspicion by anesthesiologists and surgeons, when they
are facing an intraoperative shock without a clear cause, which
does not respond to usual therapeutic protocols. Multiple trig-
gers have been reported up to date and our case identified pat-
ent blue V for sentinel lymph node biopsy as a new one.
Breast Cancer
1 3
Table1 Kounis syndrome in the operating room
Date; Study Surgery Cause Type LVEF ECG Treatment Outcome Allergies Onset ACS Onset
anaphy-
laxis
Before skin incision
2013; Sanchez Rotator cuff
arthroscopy
Rocuro-
nium ± Cefa-
zoline
I N/A ST elevation in front
and bottom of
heart
Nitroglycerine, clopidogrel,
acetylsalicylic acid
Methylprednisolone, dex-
chlorpheniramine, raniti-
dine, ephedrine
Primary PCI
Discharged No Yes Yes
2013; Shah Urologic Gelofusine II Normal ST elevations in 3
leads, pulseless
electrical activ-
ity and p-wave
asystole
Metaraminol, epinephrine,
atropine, norepinephrine
CTCA
Discharged Penicillin
Anti-tetanus
serum
Yes Yes
2015; Ates TURP Midazolam I Normal ST depression on
anterior deriva-
tions
Acetylsalicylic acid, LMWH,
atorvastatin, ranitidine
Antihistamines and corticos-
teroids
Primary PCI
Discharged No Yes No
2016; Lerner Lipoma (local
anesthesia)
Bupiv-
acaine ± lido-
caine
I 70% ST elevation in
inferior leads, ven-
tricular fibrillation
and cardiac arrest
Defibrillations, epinephrine,
amiodarone
Primary PCI
Discharged Insect stings
and oxytetra-
cycline
Yes Yes
2017; Sakamoto LAP ileocecal
excision
Rocuronium I N/A ST elevation Adrenaline, noradrenaline,
atropine, amiodarone
Steroids and antihistamines
Primary PCI
Discharged N/A Yes Yes
2018; Vasquez Foot Fracture Fentanyl ± bupiv-
acaine
I Diastolic
dysfunc-
tion
Signs of ischemia Ephedrine, dexamethasone,
salbutamol, epinephrine
Aspirin, clopidogrel, atorv-
astatin
Primary PCI
Cardio-respiratory
arrest
N/A No Yes
After skin incision
2012; Takenaka Varicose vein
stripping
Latex I Normal ST elevation Ephedrine, phenylephrine and
noradrenaline, adrenaline
Nitroglycerin, noradrenaline,
diltiazem
Discharged No Yes No

Breast Cancer
1 3
N/A non-available, LVEF left ventricular ejection fraction, ECG electrocardiogram, LAP laparoscopic, ACS acute coronary syndrome, TURP transurethral prostatectomy, TURB transurethral
resection of bladder tumor, CTCA computerized tomography coronary angiography, LMWH low molecular weight heparin, PCI percutaneous coronary intervention
Table1 (continued)
Date; Study Surgery Cause Type LVEF ECG Treatment Outcome Allergies Onset ACS Onset
anaphy-
laxis
2013; Marcoux Hernia repair Latex II 57% Third-degree
AV-block and ST-
elevations
Ephedrine, glycopyrrolate,
epinephrine, salbutamol,
norepinephrine
Clopidogrel and acetylsali-
cylic acid
Primary PCI
Coronary re-
stenosis after
4months
No Yes No
2017; Tornero Spinal surgery Succinylated
gelatin
I Lateral wall
motion
abnor-
malities
ST depression in the
left-sided leads
Ephedrine, epinephrine,
hydrocortisone, sugamma-
dex, dexamethasone
Heparin, amiodarone
Primary PCI
Discharged No No Yes
2019; Sato TURB and
LAP nephro-
ureterectomy
Cefazolin I N/A ST in lead II of the
ECG decreased
Nitroglycerin and nicorandil
Corticosteroids and chlorphe-
niramine
CTCA
Discharged No Yes No
2020; Our case Breast surgery Patent blue V I 40% ST depressions in
leads II and III
Dimetindene, corticosteroids,
norepinephrine
Clopidogrel, acetylsalicylic
acid
Primary PCI
Discharged No No Yes
Breast Cancer
1 3
Funding None to disclose for all authors.
Compliance with ethical standards
Conflict of interest The authors report no conflict of interest.
Informal consent For this type of study formal consent is not required.
References
1. Bézu C, etal. Anaphylactic response to blue dye during sentinel
lymph node biopsy. Surg Oncol. 2011;20(1):e55–e59.
2. Barthelmes L., etal. on behalf of the NEW START and ALMA-
NACH study groups. Eur J Surg oncol. 2009.
3. Montgomery LL, etal. Isosulfan blue dye reactions during
sentinel lymph node mapping for breast cancer. Anesth Analg.
2002;95(2):385–8.
4. Kounis N, Zavras G. Histamine-induced coronary artery
spasm: the concept of allergic angina. Br J Clin Pract.
1991;45(2):121–8.
5. Kounis NG. Kounis syndrome: an update on epidemiology,
pathogenesis, diagnosis and therapeutic management. Clin
Chem Lab Med (CCLM). 2016;54(10):1545–59.
6. Kounis NG, etal. Kounis syndrome: a new twist on an old
disease. Fut Cardiol. 2011;7(6):805–24.
7. Helbling A, etal. Incidence of anaphylaxis with circulatory
symptoms: a study over a 3-year period comprising 940 000
Fig. 2 Therapeutic algorithm
for perioperative Kounis
syndrome. HR heart rate, BP
blood pressure, PCI percutane-
ous coronary intervention, ICU
intensive care unit

Breast Cancer
1 3
inhabitants of the Swiss Canton Bern. Clin Exp Allergy.
2004;34(2):285–90.
8. Abdelghany M, etal. Kounis syndrome: a review article on
epidemiology, diagnostic findings, management and compli-
cations of allergic acute coronary syndrome. Int J Cardiol.
2017;232:1–4.
9. Lopez PR, Peiris AN. Kounis syndrome. South Med J.
2010;103(11):1148–55.
10. Biteker M. A new classification of Kounis syndrome. Int J Car-
diol. 2010;145(3):553.
11. Akyel A, etal. Late drug eluting stent thrombosis due to acem-
etacine: type III Kounis syndrome: kounis syndrome due to
acemetacine. Int J Cardiol. 2012;155(3):461–2.
12. Kaartinen M, Penttilä A, Kovanen PT. Accumulation of acti-
vated mast cells in the shoulder region of human coronary ath-
eroma, the predilection site of atheromatous rupture. Circula-
tion. 1994;90(4):1669–788.
13. Gázquez V, etal. Kounis syndrome: report of 5 cases. J Investig
Allergol Clin Immunol. 2010;20(2):162–5.
14. Wickman M. When allergies complicate allergies. Allergy.
2005;60:14–8.
15. Kounis NG. Kounis syndrome (allergic angina and allergic
myocardial infarction): a natural paradigm? Int J Cardiol.
2006;110(1):7–14.
16. Mazarakis A, et al. Cefuroxime-induced coronary artery
spasm manifesting as Kounis syndrome. Acta Cardiol.
2005;60(3):341–5.
17. Simons FER, etal. World allergy organization anaphylaxis
guidelines: 2013 update of the evidence base. Int Arch Allergy
Immunol. 2013;162(3):193–204.
18. Roffi M, etal. 2015 ESC Guidelines for the management of
acute coronary syndromes in patients presenting without
persistent ST-segment elevation: task force for the management
of acute coronary syndromes in patients presenting without per-
sistent ST-segment elevation of the european society of cardiol-
ogy (ESC). Eur Heart J. 2016;37(3):267–315.
19. Simons FER, et al. World allergy organization anaphy-
laxis guidelines: summary. J Allergy Clin Immunol.
2011;127(3):587–593.e22.
20. Kristensen SD, Aboyans V. 2017 ESC Guidelines for the man-
agement of acute myocardial infarction in patients presenting
with ST-segment elevation. Eur Heart J. 2018;39:119–77.
21. Barthelmes L, etal. Adverse reactions to patent blue V dye–The
NEW START and ALMANAC experience. Eur J Surg Oncol
(EJSO). 2010;36(4):399–403.
22. Paulinelli RR, etal. A prospective randomized trial comparing
patent blue and methylene blue for the detection of the sentinel
lymph node in breast cancer patients. Revista da Associação
Médica Brasileira. 2017;63(2):118–23.
23. Guo W, etal. Evaluation of the benefit of using blue dye in
addition to indocyanine green fluorescence for sentinel lymph
node biopsy in patients with breast cancer. World J Surg Oncol.
2014;12(1):290.
24. Karakatsanis A, etal. The Nordic SentiMag trial: a comparison
of super paramagnetic iron oxide (SPIO) nanoparticles versus
Tc 99 and patent blue in the detection of sentinel node (SN) in
patients with breast cancer and a meta-analysis of earlier stud-
ies. Breast Cancer Res Treat. 2016;157(2):281–94.
25. Peek MC, et al. Is blue dye still required during sentinel
lymph node biopsy for breast cancer? Ecancermedicalscience.
2016;10:674. https ://doi.org/10.3332/ecanc er.2016.674.
Publisher’s Note Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations.
