
ORIGINAL ARTICLE - VASCULAR NEUROSURGERY - ANEURYSM
Frequency and risk factors for postoperative aneurysm residual
after microsurgical clipping
Kathrin Obermueller
1
& Isabel Hostettler
1
& Arthur Wagner
1
& Tobias Boeckh-Behrens
2
& Claus Zimmer
2
&
Jens Gempt
1
& Bernhard Meyer
1
& Maria Wostrack
1
Received: 17 July 2020 / Accepted: 27 October 2020
#
The Author(s) 2020
Abstract
Objective Aneurysm residuals after clipping are a well-known problem, but the course of aneurysm remnants in follow-up is not
well studied. No standards or follow-up guidelines exist for treatment of aneurysm remnants. The aim of this study was to
evaluate the risk factors for postoperative aneurysm remnants and their changes during follow-up.
Methods We performed a retrospective analysis of 666 aneurysms treated via clipping in our hospital from 2006 to 2016.
Postoperative and follow-up angiographic data were analyzed for aneurysm remnants and regrowth. Clinical parameters and
aneurysm-specific characteristics were correlated with radiological results.
Results The frequency of aneurysm residuals was 12% (78/666). Aneurysms located in the middle cerebral artery (p =0.02)
showed a significantly lower risk for incomplete aneurysm occlusion. Larger aneurysms with a diameter of 11–25 mm (p =
0.005) showed a significantly higher risk for incomplete aneurysm occlusion. Five patients underwent re-clipping during the
same hospital stay. Remnants were stratified based on morphological characteristics into “dog ears” (n = 60) and “broad based”
(n = 13). The majority of the “dog ears” stayed stable, decreased in size, or vanished during follow-up. Broad-based remnants
showed a higher risk of regrowth.
Conclusions A middle cerebral artery location seems to lower the risk for the incomplete clip occlusion of an aneurysm. Greater
aneurysm size (11–25 mm) is associated with a postoperative aneurysm remnant. The majority of “dog-ear” remnants appear to
remain stable during follow-up. In these cases, unnecessarily frequent angiographic checks could be avoided. By contrast, broad-
based residuals show a higher risk of regrowth that requires close imaging controls if retreatment cannot be performe d
immediately.
Keywords Intracranial aneurysm
.
Aneurysm clipping
.
Aneurysm residual
.
Aneurysm remnant
.
Aneurysm regrowth
Abbreviations
ACA Anterior cerebral artery
DSA Digital subtraction angiography
Fig. Figure
FU Follow-up
H&H Hunt and Hess grade
ICA Internal carotid artery
ICD International Statistical Classification
of Diseases and Related Health Problems
ICG angiography Indocyanine green angiography
MCA Middlecerebralartery
PC Posterior circulation
SAH Subarachnoid hemorrhage
SD Standard deviation
Tab. Table
Introduction
The frequency of incomplete aneurysm occlusion after surgi-
cal clipping varies from 2–49% in different surgical series
[1–5, 7, 9, 12, 13, 15, 20, 24, 25, 27, 28, 31, 32]. The risk of
This article is part of the Topical Collection on Vascular Neurosurgery -
Aneurysm
* Kathrin Obermueller
kathrin.obermueller@tum.de
1
Department of Neurosurgery, Klinikum rechts der Isar, Technical
University Munich, Ismaninger Straße 22, 81675 Munich, Germany
2
Department of Neuroradiology, Klinikum rechts der Isar, Technical
University Munich, Ismaninger Straße 22, 81675 Munich, Germany
Acta Neurochirurgica
https://doi.org/10.1007/s00701-020-04639-5

rebleeding after aneurysm clipping is estimated at 1.3% and is
associated with the size of the residual rest [19]. However
clear criteria for retreatment and for follow-up controls of
aneurysm residuals have not yet been strictly defined.
Possible risk factors and potential follow-up dynamics are
essential for treatment decisions, as well a s for p atient
consultation.
Postoperative angiography is an accepted standard in most
neurosurgical departments, as it reveals residual filling of an-
eurysms or other complications, like major vessel occlusion
[13, 20, 27, 28]. In patients with completely clipped aneu-
rysms, further angiographic controls for the treated aneurysms
are considered unnecessary. However, no standards have been
established for follow-up (FU) in cases with residual aneu-
rysms after clipping. Currently, aneurysm remnants are cate-
gorized according t o their morphological characteristics.
David et al. and Raymond et al. classified aneurysm remnants
into 2 categories: “dog-ear” and “broad-based” residuals.
“Dog ears” consist of a small neck remnant between the parent
vessel and the base of the clip, wherea s the reconstructe d
parent vessel of “broad-based” remnants contains part of the
aneurysm wall [9, 29]. These differences in morphology sug-
gest different risks for regrowth and subsequent rebleeding.
Accordingly, the follow-up periods and the decision for sur-
gery should be made based on the estimated risk of rupture.
The aim of this study was to determine the frequency and
risk factors of residual aneurysm filling after aneurysm clip-
ping and to estimate the risk of regrowth of remnants in our
surgical series.
Methods
We performed a retrospective, single-center data analysis of
patients treated for intracranial aneurysm via clipping. The
analyzed data were collected from 2006 until 2016.
Patient inclusion/exclusion criteria
Based on the operational key for aneurysm clipping, the pa-
tient data were consecutively entered into the database. We
included patients with clipping for incidental aneurysms and
patients who were clipped for ruptured aneurysms. Patients
who received surgical treatment other than clipping (e.g.,
wrapping or trapping and bypass surgery) or, who did not
undergo postoperative digital subtrac tion angiography
(DSA) were excluded from the final analysis. Patients who
underwent aneurysm clipping in the context of other patholo-
gies (for example, arteriovenous malformation, or mycotic
aneurysms) were also excluded from the analysis. The local
ethics committees approved the study protocol (nb. 5020/11)
Interventions
Every patient received a four-vessel catheter DSA before and
a target vessel DSA within the first 24 h after surgery. The
decision for the treatment modality was based on interdisci-
plinary discussion between the neuroradiologist and neurosur-
geon. All surgeries were performed by three experienced vas-
cular neurosurgeons. For all incidental aneurysms, and partly
for ruptured aneurysms, patients underwent in traoperative
neuromonitoring comprising trans-cranially recorded motor
evoked potentials. Indocyanine green (ICG) angiography
was routinely conducted. If any aneurysm residual or vessel
occlusion was suspected in postoperative angiography, the
findings were discussed immediately to provide the possibility
of a prompt clip adjustment. Indications for FU angiography
in cases of residual filling without an indication for clip repo-
sitioning were discussed interdisciplinarily and were per-
formed depending on the size, shape, and general condition
of the patient.
Outcomes
The following clinical, radiological, and epidemiological data
were recorded for each patient: age, sex, preoperative neuro-
logical condition, Hunt and Hess grade, Fisher grade, aneu-
rysm location and size, number of aneurysms, number of op-
erations, postoperative neurological condition according to
the modified Rankin Scale, size of aneurysm remnant, size
of aneurysm remnant in FU angiography, and neurological
status during FU.
For the present research, postoperative DSA results were
screened for aneurysm remnants. An aneurysm remnant was
defined as an inflow of contrast medium with a minimum size
of 1 mm and visible on at least 2 projections of angiography in
the area of a previously described aneurysm. Follow-up ex-
aminations were reviewed for growth of the aneurysm rem-
nant, defined as any new inflow of contrast medium (com-
pared to postoperative angiography) that caused an enlarge-
ment of the residual. Changes in flow patterns or thrombosis
of the remnant were also evaluated.
Aneurysm remnants were divided according to classifica-
tion of David et al. and Raymond et al. into “dog-ear” rem-
nants with a small residual between the parent vessel and the
base of the clip and “broad-based” remnants, in which a larger
residual was still filling [9, 29]. The “dog-ear remnant” group
was scheduled for FU angiography depending on the remnant
size, the history of any previous subarachnoid hemorrhage
(SAH), and the general condition and age of the patient.
Patients with “broad-base d remnants” were either re-
operated during the same hospital stay or were designated
for FU imaging. FU DSA examinations were scheduled de-
pending on the individual risk factors and remnant character-
istics regularly within a period of 3 months to 1 year after
Acta Neurochir
surgery. The mean FU in aneurysm remnants was 31 months
(SD: ± 23.3, range 2.3–93 months).
Statistical analysis
The study sample was described using means ± standard de-
viations for the continuous variables, while categorical param-
eters were depicted using absolute and relative frequencies.
Chi-squared tests and binary logistic regression were used to
test categorical variables. Continuous variables were tested
with a t test or Wilcoxon test. A p value of 0.05 was consid-
ered statistically significant. Analyses were performed utiliz-
ing R Studio Version 1.0.4 (R Studio, Boston, USA).
Results
Data collection
In 492 patients, a total of 666 ruptured and unruptured aneu-
rysms were treated between April 2006 and December 2016.
The reasons for case exclusion are listed in Fig. 1.
In 18 patients, no postoperative DSA was performed. In
this group, 11 cases had suffered a severe SAH and had died
soon after aneurysm clipping. In 3 cases, other medical cir-
cumstances (malignant disease) were treated immediately and
were judged more important than the aneurysm.
Complications during the initial angiography, with arterial
dissection in 2 and symptomatic arterial emboli in 1 case,
led to no postoperative DSA. One patient rejected postopera-
tive DSA after elective clipping.
Patient data
Patient data and data on the treated aneurysms are shown in
Tables 1 and 2.
Frequency of postoperative aneurysm remnants
Residual filling was found in 78/666 aneurysms (12%). Data
on patients with postoperative aneurysm residuals are present-
ed in Table 3.
Risk of aneurysm residuals
Binary logistic regression revealed a higher risk of an aneu-
rysm residual for aneurysms sized 11–25 mm, with an odds
ratio of 9.36 (CI 1.96–44.61; p = 0.005). A location in the
MCA showed a significantly lower risk for aneurysm resid-
uals when compared with other locations, with an odds ratio
of 0.31 (CI: 0.12–0.83; p = 0.02) (Table 4). Sex, number of
aneurysms per patient, and emergency setting of surgery
showed no significant association with postoperative aneu-
rysm residuals.
Follow-up of aneurysm remnants
Figure 2 shows the classification and early treatment of aneu-
rysm remnants. FU was available for 33/60 (55%) aneurysms
in the dog-ear group and for 7/13 (54%) in the group of broad-
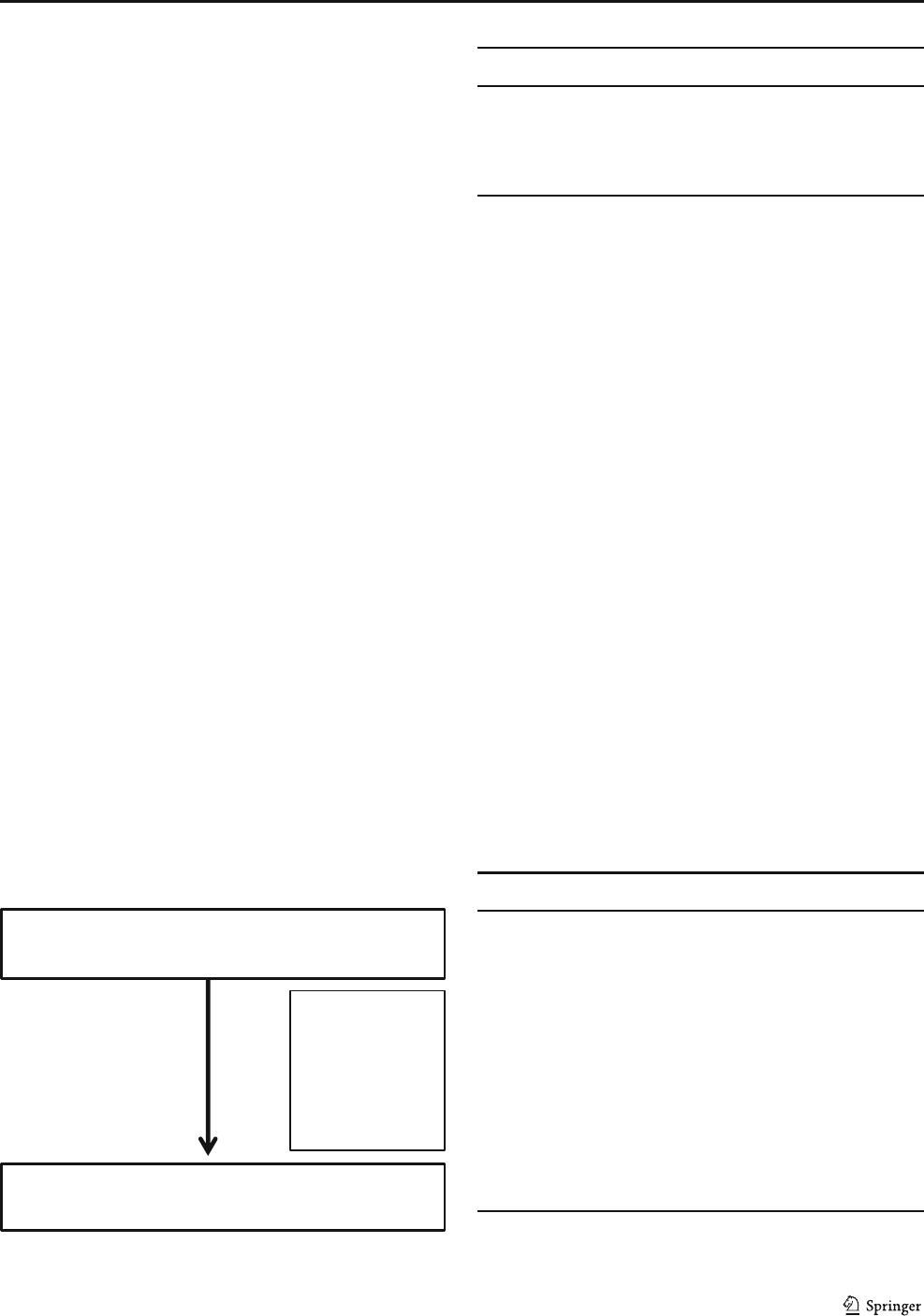
700 aneurysm-clipping procedures identified
via ICD I67.1
666 aneurysm-clipping procedures
34 excluded
No postoperative DSA: 18
Surgical technique other than
clipping used: 6
Clipping not feasible: 4
No aneurysm identified: 2
Aneurysm associated with
other pathology: 5
Fig. 1 Flowchart of procedure inclusion via ICD code I67.1
Table 1 Patient data and clinical characteristics (n =492)
Parameter Value
Age at surgery mean ± SD 55.48 ± 13.1
Gender female n, (%) 343 (70)
Acute SAH n, (%) 252 (51)
Multiple aneurysms ≥ 2 n, (%) 167 (34)
SAH, subarachnoid hemorrhage
Table 2 Data on treated aneurysms (n =666)
Parameter Value
Aneurysm location n,(%)
ACA 152 (23)
ICA 140 (21)
MCA 340 (51)
PC 34 (5)
Aneurysm size n,(%)
< 3 mm 136 (20.4)
3–6 mm 306 (46.0)
7–10 mm 129 (19.4)
11–25 mm 59 (8.8)
>25mm 4(0.6)
Residual of coiled aneurysm 32 (4.8)
ACA, anterior cerebral artery; ICA, interior carotid artery; MCA, middle
cerebral artery; PC, posterior circulation
Acta Neurochir
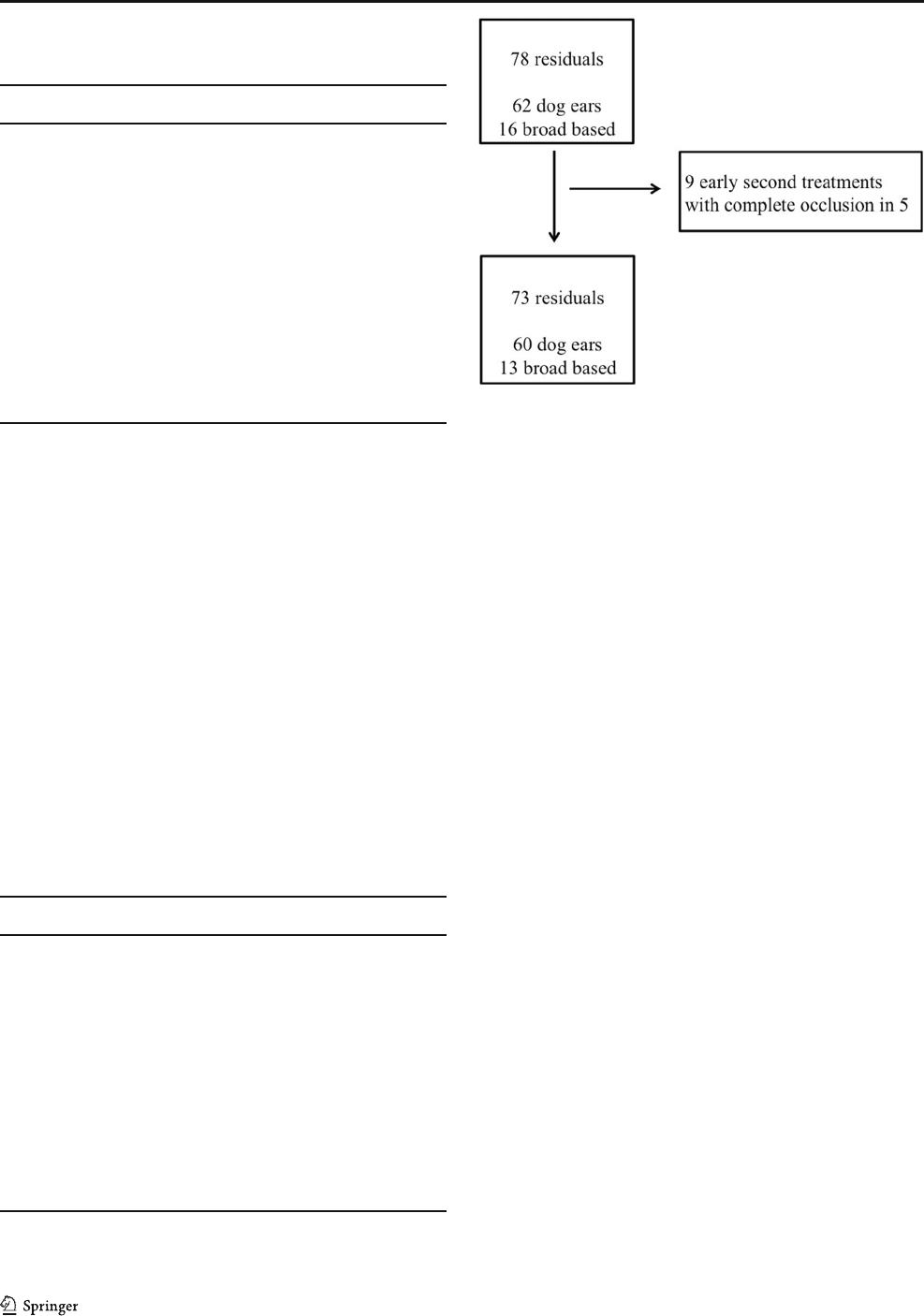
based residuals. The mean FU was 31 months (SD: ± 23.3,
range 2.3–93 months).
The reasons for missing FU differed. In 12 cases, FU an-
giography was recommended but the patient did not attend the
FU examination. In 7 cases, the patients were in poor neuro-
logical condition and FU angiography was recommended on-
ly if the patients improved during their postoperative courses.
These were all patients who had suffered a severe SAH. In 4
dog-ear cases, FU angiography was not recommended
because of the small size of the aneurysm remnant. In 9 cases,
a FU recommendation was not specified at discharge.
Follow-up of dog-ear remnants and broad-based remnants
Table 5 shows the course of dog-ear residuals during FU.
The major par t of the dog-ear residuals stayed stable, de-
creased in size, or closed during FU (94%). An increase in
size was recorded in two cases.
During FU, two of the broad-based aneurysms increased in
size, one decreased, and none closed spontaneously. The num-
ber of broad-bas ed aneurysms i n FU was small ( n =7)
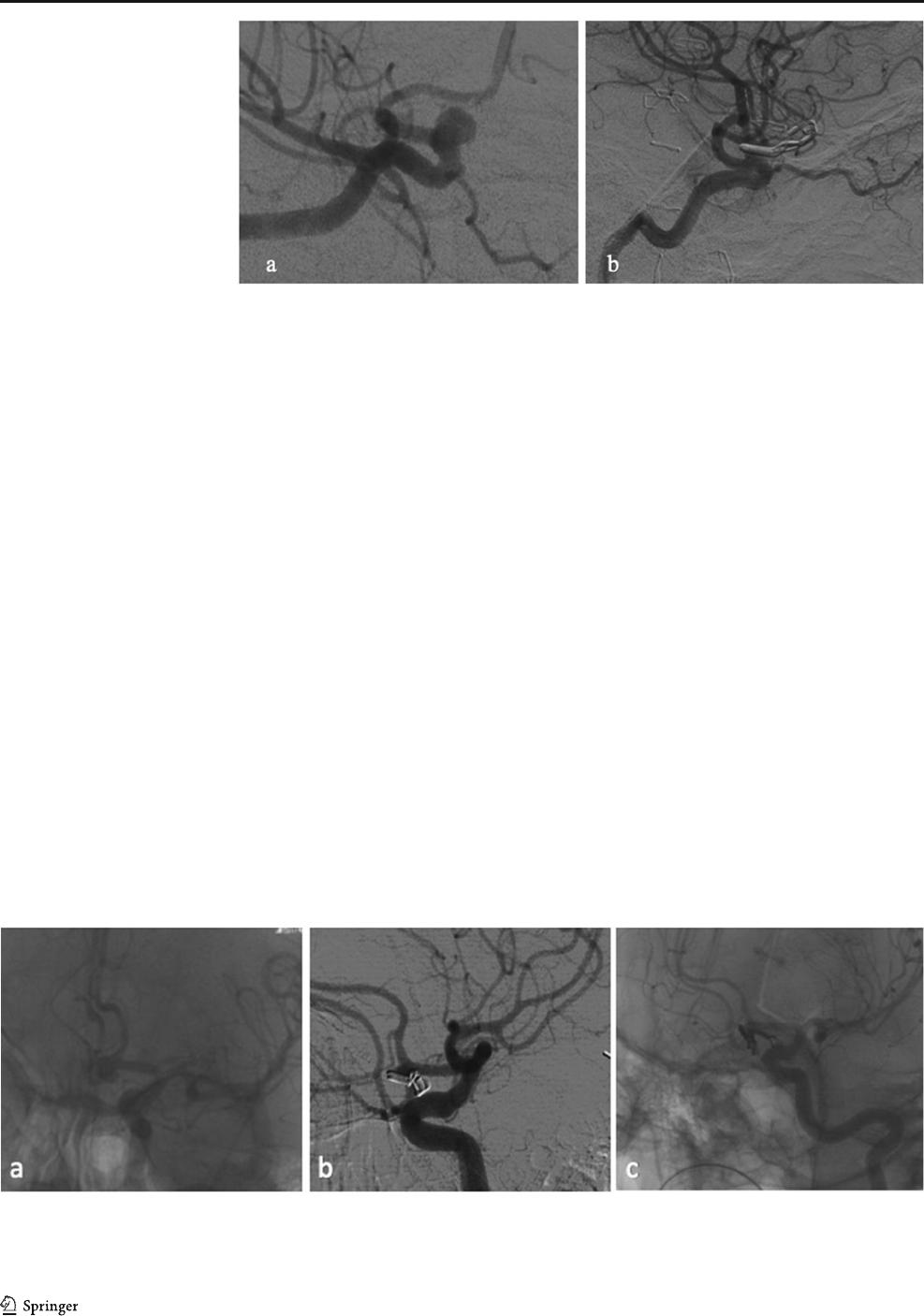
(Table 6). Figures 3 and 4 show typical DSA findings of a
dog ear and a broad-based aneurysm residual.
Treatment of aneurysm residuals during follow-up
All aneurysm remnants with increasing size were treated (2 in
the dog-ear group, 2 in the broad-based group). The 2 progres-
sive dog-ear residuals were treated 1 year after clipping by
coiling and stent-assisted coiling. The progressive broad-
based residuals were treated via clipping in one case at 6
months after the first surgery and by coiling in the other case
at 1 year after surgery.
Two broad-based aneurysm residuals were treated irrespec-
tive of the residual growth during the FU. In 1 case, the broad-
based residual of a paraophthalmic aneurysm was treated with
a flow diverter 6 months postoperatively. In another case, a
paraophthalmic ICA aneurysm was clipped and postoperative
angiography revealed a small remnant. Early second surgery
was performed, but a postoperative DSA still showed an an-
eurysm residual. One year later, the aneurysm residual was
treated with stent-assisted coiling and showed no further
growth during FU.
Fig. 2 Flowchart of aneurysm residuals
Table 4 Factors associated with postoperative aneurysm residuals. The
factor positively associated with postoperative aneurysm residuals is
aneurysm size 11–25 mm. The location of the “MCA” aneurysm is asso-
ciated with a reduced risk for postoperative aneurysm residuals. (Binary
logistic regression)
Parameter OR (95%CI) p
Aneurysm location
ACA 0.67 (0.24–1.84) 0.44
ICA 1.35 (0.51–3.61) 0.55
MCA 0.31 (0.12–0.83) 0.02
Aneurysm size n (%)
< 3 mm 0.98 (0.19–4.99) 0.98
3–6 mm 2.04 (0.46–9.12) 0.35
7–10 mm 4.23 (0.95–20.60) 0.06
11–25 mm 9.36 (1.96–44.61) 0.005
>25mm 4.46(0.37–66.74) 0.28
Surgery in acute phase 0.67 (0.38–1.16) 0.16
ACA, anterior cerebral artery; ICA, interior carotid artery; MCA, middle
cerebral artery
Table 3 Data on aneurysms with residual filling in postoperative DSA
(n = 78). The percentage is based on the total number of operated patients
in the respective subgroup
Parameter Value
Aneurysm location n, (% of the entire subgroup)
ACA (n =152) 18(11)
ICA (n =140) 28(20)
MCA (n =340) 25(7)
PC (n =34) 8(24)
Aneurysm size n,(%)
< 3 mm (n = 136) 7 (5)
3–6mm(n =306) 28(9)
7–10 mm (n =129) 23(17)
11–25 mm (n = 59) 18 (31)
>25mm(n = 4) 1 (25)
Residual of coiled aneurysm (n =32) 2(6)
ACA, anterior cerebral artery; ICA, interior carotid artery; MCA, middle
cerebral artery; PC, posterior circulation
Acta Neurochir

Discussion
In the current study, we analyzed the risk factors for aneurysm
remnants after microsurgical clipping and the course of aneu-
rysm remnants in FU. Postoperative angiography revealed a
rate of 12% for residual aneurysm filling in clipped aneurysms
in our series. These data are consistent with other published
studies, as the frequency of residual filling of aneurysms after
clipping is reported to range from 2.3 to 19.3%. One series
from Korea revealed an incidence of residual filling of even
49% when controlled with 3D angiography and defining a
remnant size from 1 mm onward [1–5, 7, 9, 12, 13, 15, 20,
24, 25, 27, 28, 31, 32]. Our series confirms the need for early
postoperative angiography to certify the success of the surgery
and, in the c ase of remn ants, to ensure that FU or further
therapy can be scheduled appropriately. The necessit y for
postoperative angiography has been discussed previously [8,
10, 12], and this procedure remains the absolute standard for
control after aneurysm surgery.
Risk factors for incomplete aneurysm occlusion
A greater aneurysm size (11–25 mm) was associated with
incomplete surgical occlusion. Jabbarli et al. described similar
results for aneurysms sized > 12 mm and an association with a
higher risk for a clip remnant [18].
The location of the aneurysm is considered important for
the occurrence of an aneurysm remnant, as the risk of aneu-
rysm remnants seem to be greater for paraophthalmic, anterior
communicating artery, and basilar artery aneurysms [18, 27,
32]. Our series revealed a lower risk for aneurysm residuals
following clipping of MCA aneurysms, in agreement with
previously described findings and reflecting the technically
easier accessibility as compared to paraophthalmic and poste-
rior circulation aneurysms [21, 28].
Direct intraoperative catheter angiography within a hybrid
operating room is described as potentially beneficial for the
occlusion rates of the aneurysms [22, 26]. However, in our
series, only 5 patients underwent early clip revision surgery.
This was primarily due to the technically difficult
configuration of the aneurysm; therefore, it could not be
avoided with the use of intraoperative DSA.
Angiographic and clinical follow-up of aneurysm remnants
In our series, the major part of dog-ear residuals remained
stable, decreased in size, or vanished during the FU.
Therefore, foregoing angiographic controls might be justified,
particularly in patients without risk factors such as a previous
history of SAH, hereditary or de novo aneurysms, hypertonus,
or smoking. A younger age should also be regarded as a po-
tential risk factor because of the previously reported increased
risk of regrowth of aneurysm residuals in patients under 45
years old [18].
In 2004, Akyüz et al. performed a long-term angiographic
FU (median 46.6 months) of 166 clipped aneurysms, with 7
residuals (4.2%), using the Sindou residual grading system.
They reported 5 small neck residuals and 2 with a broader
residual. One case of regrowth was recorded in a small rem-
nant and led to reoperation, but no cases of regrowth occurred
among the larger remnants. These surgeons also had one case
of spontaneous thrombosis of a small remnant but experienced
no cases of rebleeding during FU. They concluded that small
aneurysm remnants may stay stable [3].
The FU of broad-based aneurysms in our series was incom-
plete, but it revealed a high rate of regrowth (28%). This was
also observed by David et al., who noted aneurysm regrowth
in 3 out of 4 cases [9]. Only one broad-based residual de-
creased in size during FU, but it did not close completely.
Broad-based residuals seem to have a high risk of regrowth
and should be scheduled for FU, as the need for retreatment
seems to occur frequently. Furthermore, the CARAT study
revealed a size-dependent risk of rebleeding in clipped and
coiled aneurysms [19].
In treated aneurysms after SAH, the risk of rehemorrhage
has been estimated as 0.11–0.21% in the first year for coiled
aneurysms and 0.0–0.03% for clipped aneurysms [10, 19]. In
our series, no case of rebleeding occurred in the incompletely
clipped aneurysms within the F U of a maximum o f 93
months. David et al. estimated the risk of rebleeding in aneu-
rysms with postoperative remnants as 1.5% per year [9]. A
series of 715 surgically treated patients reported by Feuerberg
Table 5 Behavior of dog-ear re-
siduals in follow-up (n =33)
Stable size in
FU
Decreased size in
FU
Closed in
FU
Increased in
size
Number of aneurysm residuals n,
(%)
22 (67) 3 (9) 6(18) 2 (6)
Table 6 Behavior of broad-based
residuals in follow-up (n =7)
Stable size in FU Decreased size in FU Increased in size
Number of aneurysm residuals n, (%) 4 (60) 1 (14) 2 (28)
Acta Neurochir
et al. revealed a risk of rebleeding in aneurysm residuals of
0.38–0.79% per year, leading to a discussion of whether this
risk of rebleeding might justify the risk of reoperation [15].
Rauzzino et al. determined a relatively high risk of rebleeding
in aneurysm remnants. Surgery in 312 aneurysms led to a
remnant rate of 4.2% (n = 13). Only 4 of these remnants were
not treated immediately after initial surgery. All 4 of these
remaining aneurysm remnants became symptomatic within 2
years: 3 with a new SAH and 1 because of a local mass effect
[28]. Configuration of the remnants was not further specified
in this study.
Furthermore, radiation exposure, periprocedural complica-
tions, and costs should also be factored into the decision for
invasive angiographic controls. The development of less in-
vasive but adequate imaging procedures with lower radiation
exposure for the patient, such as metal artifact reduced MRI,
would be desirable. However, even the most recent, newly
developed MRI sequences are not able to suppress the artifacts
so strongly to be able to determine the fine differences in size
or configuration of the neck remnant [16]. The MRI is nowa-
days much more useful in checking untreated aneurysms or in
excluding de novo formations in predisposed patients.
CT angiography (CT-A) is less invasive and has been com-
pared to classical DSA in different studies. The sensitivity for
detection of postoperative aneurysm remnants varied between
50–100% comparing CT-A to DSA [6, 11, 14, 23, 30, 33, 34].
The precision of the CT-A strongly decreases with the usage
of multiple clips, in cases with posterior circulation aneurysms
and in small remnants.
Sagara et al. came to the conclusion that the usage of mul-
tiple clips is an indication for 3D DSA [30]. Bharatha et al.
reported a sensitivity of 88% for the detection of aneurysm
remnants after clipping when comparing DSA to CT-A,
whereas the sensitivity for a small neck remnant (mean size
1 mm) was only 20% [6].
Dundar et al. compared traditional DSA to subtraction CT-A
and came to the conclusion that DSA remains the gold standard
as residuals < 3 mm are not reliably examined with CT-A [14].
In our opinion and according to previous publications, CT-A
might be suitable, for long-term follow-up controls when DSA
controls were initially stable and CT-A quality is good [17].
Study limitations
One limitation of our study is its retrospective data collection.
Furthermore, FU is not available for almost half the patients
with incompletely occluded aneurysms, either due to the poor
clinical condition of certain SAH patients or due to the esti-
mated low-risk factors based on patient’s postoperative angi-
ography and medical history. This may obscure the true data
Fig. 4 a Preoperative DSA of an Acoma aneurysm in a patient with SAH
H&H II; b postoperative DSA 1 showed a broad-based aneurysm residual
and the patient underwent revision surg ery on the same d ay; c
postoperative DSA 2 demonstrates now complete occlusion of the resid-
ual, which was achieved by placement of further clips
Fig. 3 a Preoperative DSA
demonstrating an incidental
paraophthalmic ICA aneurysm
which was scheduled for clipping;
b postoperative DSA reveals
small dog-ear residual. This pa-
tient was scheduled for follow-up
DSA, which showed a stable
result
Acta Neurochir

concerning growth dynamics of incompletely occluded
aneurysms.
Conclusions
MCA aneurysms appear to have a lower risk for postoperative
aneurysm residuals than is observed for an aneurysm in other
locations. A large part of dog-ear residuals appear to remain
stable, decrease in size, or close during FU. Management of
aneurysm remnants should consider individual risk factors
and the remnant configuration.
Funding Open Access funding enabled and organized by Projekt DEAL.
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of
interest.
Ethical approval All procedures performed in studies involving human
participants were in accordance with the ethical standards of the institu-
tional and/or national research committee and with the 1964 Helsinki
declaration and its later amendments or comparable ethical standards.
Open Access This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adap-
tation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, pro-
vide a link to the Creative Commons licence, and indicate if changes were
made. The images or other third party material in this article are included
in the article's Creative Commons licence, unless indicated otherwise in a
credit line to the mat erial. If material is not included in the article's
Creative Commons licence and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/.
References
1. Acevedo JC, Turjman F, Sindou M (1997) Postoperative arteriog-
raphy in surgery for intracranial aneurysm. Prospective study in a
consecutive series of 267 operated aneurysms. Neuro-Chirurgie 43:
275–284
2. Ahn SS, Kim YD (2010) Three-dimensional digital subtraction
angiographic evaluation of aneurysm remnants after clip placement.
J Korean Neurosurg Soc 47:185–190. https://doi.org/10.3340/jkns.
2010.47.3.185
3. Akyuz M, Tuncer R, Yilmaz S, Sindel T (2004) Angiographic
follow-up after surgical treatment of intracranial aneurysms. Acta
Neurochir 146:245–250; discussion 250. https://doi.org/10.1007/
s00701-003-0206-z
4. Allcock JM, Drake CG (1963) Postoperative angiography in cases
of ruptured intracranial aneurysm. J Neurosurg 20:752–759. https://
doi.org/10.3171/jns.1963.20.9.0752
5. Asgari S, Doerfler A, Wanke I, Schoch B, Forsting M, Stolke D
(2002) Complementary management of partially occluded
aneurysms by using surgical or endovascular therapy. J
Neurosurg 97:843–850. https://doi.org/10.3171/jns.2002.97.4.
0843
6. Bharatha A, Yeung R, Durant D, Fox AJ, Aviv RI, Howard P,
Thompson AL, Bartlett ES, Symons SP (2010) Comparison of
computed tomography angiography with digital subtraction angi-
ography in the assessment of clipped intracranial aneurysms. J
Comput Assist Tomogr 34:440–445. https://doi.org/10.1097/RCT.
0b013e3181d27393
7. Choi SW, Ahn JS, Park JC, Kwon do H, Kwun BD, Kim CJ (2012)
Surgical treatment of unruptured intracranial middle cerebral artery
aneurysms: angiographic and clinical outcomes in 143 aneurysms. J
Cerebrovasc Endovasc Neurosurg 14:289–294. https://doi.org/10.
7461/jcen.2012.14.4.289
8. Cronk K, Spetzler RF (2010) Commentary for recurrent intracranial
aneurysms after successful neck clipping. World Neurosurg 74:
437–438. https://doi.org/10.1016/j.wneu.2010.10.032
9. David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT,
Partovi S (1999) Late angiographic follow-up review of surgically
treated aneurysms. J Neurosurg 91:396–401. https://doi.org/10.
3171/jns.1999.91.3.0396
10. de Sousa AA (2010) Follow-up of cerebral aneurysms after neck
clipping. World Neurosurg 74:435–436. https://doi.org/10.1016/j.
wneu.2010.08.003
11. Dehdashti AR, Binaghi S, Uske A, Regli L (2006) Comparison of
multislice computerized tomography angiography and digital sub-
traction angiography in the postoperative evaluation of patients
with clipped aneurysms. J Neurosurg 104:395–403. https://doi.
org/10.3171/jns.2006.104.3.395
12. Drake CG, Allcock JM (1973) Postoperative angiography and the
“slipped” clip. J Neurosurg 39:683–689. https://doi.org/10.3171/
jns.1973.39.6.0683
13. Drake CG, Friedman AH, Peerless SJ (1984) Failed aneurysm sur-
gery. Reoperation in 115 cases. J Neurosurg 61:848–856. https://
doi.org/10.3171/jns.1984.61.5.0848
14. Dundar TT, Aralasmak A, Kitiş S, Yılmaz FT, Abdallah A (2019)
Comparison of subtracted computed tomography from computed
tomography perfusion and digital subtraction angiography in resi-
due evaluation of treated intracranial aneurysms. World Neurosurg
132:e746–e751. https://doi.org/10.1016/j.wneu.2019.08.028
15. Feuerberg I, Lindquist C, Lindqvist M, Steiner L (1987) Natural
history of postoperative aneurysm rests. J Neurosurg 66:30–34.
https://doi.org/10.3171/jns.1987.66.1.0030
16. Friedrich B, Wostrack M, Ringel F, Ryang YM, Forschler A, Waldt
S, Zimmer C, Nittka M, Preibisch C (2016) Novel metal artifact
reduction techniques with use of slice-encoding metal artifact cor-
rection and view-angle tilting mr imaging for improved visualiza-
tion of brain t issue near intracrania l aneurysm clips. Cl in
Neuroradiol 26:31–37. https://doi.org/10.1007/s00062-014-0324-4
17. Gölitz P, Struffert T, Ganslandt O, Saake M, Lücking H, Rösch J,
Knossalla F, Doerfler A (2012) Optimized angiographic computed
tomography with intravenous contrast injection: an alternative to
conventional angiography in the follow-up of clipped aneurysms?
J Neurosurg 117:29–36. https://doi.org/10.3171/2012.3.Jns111895
18. Jabbarli R, Pierscianek D, Wrede K, Dammann P, Schlamann M,
Forsting M, Muller O, Sure U (2016) Aneurysm remnant after
clipping: the risks and consequences. J Neurosurg 125(5):1249–
1255. https://doi.org/10.3171/2015.10.jns151536
19. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler
GR, Gress DR, Investigators C (2008) Predictors of rehemorrhage
after treatment of ruptured intracranial aneurysms: the Cerebral
Aneurysm Rerupture After Treatment (CARAT) study. Stroke 39:
120–125. https://doi.org/10.1161/STROKEAHA.107.495747
20. Kang HS, Han MH, Kwon BJ, Jung SI, Oh CW, Han DH, Chang
KH (2004) Postoperative 3D angiography in intracranial aneu-
rysms. AJNR Am J Neuroradiol 25:1463–1469
Acta Neurochir

21. Kivisaari RP, Porras M, Ohman J, Siironen J, Ishii K, Hernesniemi
J (2004) Routine cerebral angiography after surgery for saccular
aneurysms: is it worth it? Neurosurgery 55:1015–1024
22. Klopfenstein JD, Spetzler RF, Kim LJ, Feiz-Erfan I, Han PP,
Zabra mski JM, Porter RW , Albuquerque FC, McDougall CG,
Fiorella DJ (2004) Comparison of routine and selective use of in-
traoperative angiography during aneurysm surgery: a prospective
assessment. J Neurosurg 100:230–235. https://doi.org/10.3171/jns.
2004.100.2.0230
23. Kunert P, Prokopienko M, Gola M, Dziedzic T, Jaworski M,
Marchel A (2013) Assessment of long-term results of intracranial
aneurysm clipping by means of computed tomography angiogra-
phy. Neurol Neurochir Pol 47:18–26. https://doi.org/10.5114/ninp.
2012.31549
24. Le Roux PD, Elliott JP, Eskridge JM, Cohen W, Winn HR (1998)
Risks and benefits of diagnostic angiography after aneurysm sur-
gery: a retrospective analysis of 597 studies. Neurosurgery 42:
1248–1254 discussion 1254-1245
25. Macdonald RL, Wallace MC, Kestle JR (1993) Role of angiogra-
phy following aneurysm surgery. J Neurosurg 79:826–832. https://
doi.org/10.3171/jns.1993.79.6.0826
26. Marbacher S, Mendelowitsch I, Gruter BE, Diepers M, Remonda L,
Fandino J (2018) Comparison of 3D intraoperative digital subtrac-
tion angiography and intraoperative indocyanine green video angi-
ography during intracranial aneurysm surgery. J Neurosurg 131(1):
64–71. https://doi.org/10.3171/2018.1.jns172253
27. Meyer B, Urbach H, Schaller C, Baslam M, Nordblom J, Schramm
J (2004) Immediate postoperative angiography after aneury sm
clipping–implications for quality control and guidance of further
management. Zentralbl Neurochir 65:49–56. https://doi.org/10.
1055/s-2004-816267
28. Rauzzino MJ, Quinn CM, Fisher WS 3rd (1998) Angiography after
aneurysm surgery: indications for “selective” angiography. Surg
Neurol 49:32–40 discussion 40-31
29. Raymond J, Roy D, Bojanowski M, Moumdjian R, L’Espérance G
(1997) Endovascular treatment of acutely ruptured and unruptured
aneurysms of the basilar bifurcation. J Neurosurg 86:211–219.
https://doi.org/10.3171/jns.1997.86.2.0211
30. Sagara Y, Kiyosue H, Hori Y, Sainoo M, Nagatomi H, Mori H
(2005) Limitations of three-dimensional reconstructed computer-
ized tomography angiography after clip placement for intracranial
aneurysms. J Neurosurg 103:656–661. https://doi.org/10.3171/jns.
2005.103.4.0656
31. Sato S, Suzuki J (1971) Prognosis in cases of intracranial aneurysm
after incomplete direct operations. Acta Neurochir 24:245–252
32. Sindou M, Acevedo JC, Turjman F (1998) Aneurysmal remnants
after microsurgical clipping: classification and results from a pro-
spective angiographic study (in a consecutive series of 305 operated
intracranial aneurysms). Acta Neurochir 140:1153–1159
33. Teksam M, McKinney A, Casey S, Asis M, Kieffer S, Truwit CL
(2004) Multi-section CT angiography for detection of cerebral an-
eurysms. AJNR Am J Neuroradiol 25:1485–1492
34. van Loon JJ, Yousry TA, Fink U, Seelos KC, Reulen HJ, Steiger HJ
(1997) Postoperative spiral computed tomography and magnetic
resonance angiography after aneurysm clipping with titanium clips.
Neurosurgery 41:851–856; discussion 856-857. https://doi.org/10.
1097/00006123-199710000-00016
Publisher’snoteSpringer Nature remains neutral with regard to jurisdic-
tional claims in published maps and institutional affiliations.
Acta Neurochir
